Bench-to-bedside review: circulating microparticles--a new player in sepsis?
- PMID: 21067540
- PMCID: PMC3219244
- DOI: 10.1186/cc9231
Bench-to-bedside review: circulating microparticles--a new player in sepsis?
Abstract
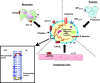
In sepsis, inflammation and thrombosis are both the cause and the result of interactions between circulating (for example, leukocytes and platelets), endothelial and smooth muscle cells. Microparticles are proinflammatory and procoagulant fragments originating from plasma membrane generated after cellular activation and released in body fluids. In the vessel, they constitute a pool of bioactive effectors pulled from diverse cellular origins and may act as intercellular messengers. Microparticles expose phosphatidylserine, a procoagulant phospholipid made accessible after membrane remodelling, and tissue factor, the initiator of blood coagulation at the endothelial and leukocyte surface. They constitute a secretion pathway for IL-1β and up-regulate the proinflammatory response of target cells. Microparticles circulate at low levels in healthy individuals, but undergo phenotypic and quantitative changes that could play a pathophysiological role in inflammatory diseases. Microparticles may participate in the pathogenesis of sepsis through multiple ways. They are able to regulate vascular tone and are potent vascular proinflammatory and procoagulant mediators. Microparticles' abilities are of increasing interest in deciphering the mechanisms underlying the multiple organ dysfunction of septic shock.
Figures


References
-
- Zwaal RF, Schroit AJ. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood. 1997;89:1121–1132. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

