Identification of a stem-like cell population by exposing metastatic breast cancer cell lines to repetitive cycles of hypoxia and reoxygenation
- PMID: 21067584
- PMCID: PMC3046435
- DOI: 10.1186/bcr2773
Identification of a stem-like cell population by exposing metastatic breast cancer cell lines to repetitive cycles of hypoxia and reoxygenation
Abstract
Introduction: The irregular vasculature of solid tumors creates hypoxic regions, which are characterized by cyclic periods of hypoxia and reoxygenation. Accumulated evidence suggests that chronic and repetitive exposure to hypoxia and reoxygenation seem to provide an advantage to tumor growth. Although the development of hypoxia tolerance in tumors predicts poor prognosis, mechanisms contributing to hypoxia tolerance remain to be elucidated. Recent studies have described a subpopulation of cancer stem cells (CSC) within tumors, which have stem-like properties such as self-renewal and the ability to differentiate into multiple cell types. The cancer stem cell theory suggests CSCs persist in tumors as a distinct population and cause relapse and metastasis by giving rise to new tumors. Since hypoxia is considered to be one of the critical niche factors to promote invasive growth of tumors, we hypothesize that repetitive cycles of hypoxia/reoxygenation also play a role in the enrichment of breast CSCs.
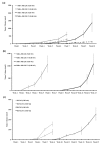
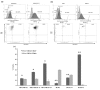
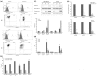
Methods: Two metastatic human breast cancer cell lines (MDA-MB 231 and BCM2) were used to optimize the conditions of hypoxia and reoxygenation cycles. The percentage of CSCs in the cycling hypoxia selected subpopulation was analyzed based on the CD44, CD24, ESA, and E-cadherin expression by three-color flow cytometry. Colony formation assays were used to assess the ability of this subpopulation to self-renew. Limiting dilution assays were performed to evaluate the tumor-initiating and metastatic ability of this subpopulation. Induction of EMT was examined by the expression of EMT-associated markers and EMT-associated microRNAs.
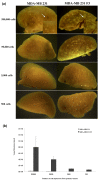
Results: Using an optimized hypoxia and reoxygenation regimen, we identified a novel cycling hypoxia-selected subpopulation from human breast cancer cell lines and demonstrated that a stem-like breast cancer cell subpopulation could be expanded through repetitive hypoxia/reoxygenation cycles without genetic manipulation. We also found that cells derived from this novel subpopulation form colonies readily, are highly tumorigenic in immune-deficient mice, and exhibit both stem-like and EMT phenotypes.
Conclusions: These results provide the validity to the newly developed hypoxia/reoxygenation culture system for examining the regulation of CSCs in breast cancer cell lines by niche factors in the tumor microenvironment and developing differential targeting strategies to eradicate breast CSCs.
Figures


Similar articles
-
Prolonged drug selection of breast cancer cells and enrichment of cancer stem cell characteristics.J Natl Cancer Inst. 2010 Nov 3;102(21):1637-52. doi: 10.1093/jnci/djq361. Epub 2010 Oct 8. J Natl Cancer Inst. 2010. PMID: 20935265 Free PMC article.
-
SLUG/SNAI2 and tumor necrosis factor generate breast cells with CD44+/CD24- phenotype.BMC Cancer. 2010 Aug 6;10:411. doi: 10.1186/1471-2407-10-411. BMC Cancer. 2010. PMID: 20691079 Free PMC article.
-
Multiple lineages of human breast cancer stem/progenitor cells identified by profiling with stem cell markers.PLoS One. 2009 Dec 21;4(12):e8377. doi: 10.1371/journal.pone.0008377. PLoS One. 2009. PMID: 20027313 Free PMC article.
-
Breast cancer stem cells and intrinsic subtypes: controversies rage on.Curr Stem Cell Res Ther. 2009 Jan;4(1):50-60. doi: 10.2174/157488809787169110. Curr Stem Cell Res Ther. 2009. PMID: 19149630 Review.
-
The evolving concept of cancer stem-like cells in thyroid cancer and other solid tumors.Lab Invest. 2017 Oct;97(10):1142-1151. doi: 10.1038/labinvest.2017.41. Epub 2017 Apr 10. Lab Invest. 2017. PMID: 28394318 Review.
Cited by
-
Transcriptional shift identifies a set of genes driving breast cancer chemoresistance.PLoS One. 2013;8(1):e53983. doi: 10.1371/journal.pone.0053983. Epub 2013 Jan 10. PLoS One. 2013. PMID: 23326553 Free PMC article.
-
Cancer stem cells, tumor dormancy, and metastasis.Front Endocrinol (Lausanne). 2012 Oct 23;3:125. doi: 10.3389/fendo.2012.00125. eCollection 2012. Front Endocrinol (Lausanne). 2012. PMID: 23109929 Free PMC article.
-
Hypoxia-inducible factor 1α promotes primary tumor growth and tumor-initiating cell activity in breast cancer.Breast Cancer Res. 2012 Jan 7;14(1):R6. doi: 10.1186/bcr3087. Breast Cancer Res. 2012. PMID: 22225988 Free PMC article.
-
Hypoxia in solid tumors: a key promoter of cancer stem cell (CSC) resistance.J Cancer Res Clin Oncol. 2020 Jan;146(1):19-31. doi: 10.1007/s00432-019-03080-1. Epub 2019 Nov 16. J Cancer Res Clin Oncol. 2020. PMID: 31734836 Free PMC article. Review.
-
Breast cancer stem cell: the roles and therapeutic implications.Cell Mol Life Sci. 2017 Mar;74(6):951-966. doi: 10.1007/s00018-016-2334-7. Epub 2016 Aug 16. Cell Mol Life Sci. 2017. PMID: 27530548 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

