Laminin-511 and integrin beta-1 in hair follicle development and basal cell carcinoma formation
- PMID: 21067603
- PMCID: PMC2995472
- DOI: 10.1186/1471-213X-10-112
Laminin-511 and integrin beta-1 in hair follicle development and basal cell carcinoma formation
Abstract
Background: Initiation of the hair follicle placode and its subsequent growth, maturation and cycling in post-natal skin requires signaling interactions between epithelial cells and adjacent dermal cells and involves Shh signaling via the primary cilium. Previous reports have implicated laminins in hair follicle epithelial invagination.
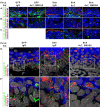
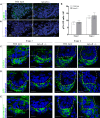
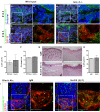
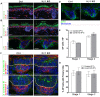
Results: Here we use a human BCC model system and mouse mutants to re-evaluate the role of laminin-511 in epithelial invagination in the skin. Blocking laminin 511 and 332 in BCCs maintains primary cilia and Shh signalling, but prevents invagination. Similarly, in laminin-511 and dermal beta-1 integrin mutants, dermal papilla development and primary cilia formation are normal. Dermal beta-1 integrin mutants have normal hair follicle development.
Conclusions: Our data provides support for a primary role of laminin-511 promoting hair follicle epithelial downgrowth without affecting dermal primary cilia and Shh target gene induction.
Figures


References
-
- Van Scott EJ, Reinertson RP. The modulating influence of stromal environment on epithelial cells studied in human autotransplants. J Invest Dermatol. 1961;36:109–31. - PubMed
-
- Aszterbaum M, Rothman A, Johnson RL, Fisher M, Xie J, Bonifas JM, Zhang X, Scott MP, Epstein EH Jr. Identification of mutations in the human PATCHED gene in sporadic basal cell carcinomas and in patients with the basal cell nevus syndrome. J Invest Dermatol. 1998;110:885–8. doi: 10.1046/j.1523-1747.1998.00222.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

