Abnormal iron metabolism in fibroblasts from a patient with the neurodegenerative disease hereditary ferritinopathy
- PMID: 21067605
- PMCID: PMC2993710
- DOI: 10.1186/1750-1326-5-50
Abnormal iron metabolism in fibroblasts from a patient with the neurodegenerative disease hereditary ferritinopathy
Abstract
Background: Nucleotide duplications in exon 4 of the ferritin light polypeptide (FTL) gene cause the autosomal dominant neurodegenerative disease neuroferritinopathy or hereditary ferritinopathy (HF). Pathologic examination of patients with HF has shown abnormal ferritin and iron accumulation in neurons and glia in the central nervous system (CNS) as well as in cells of other organ systems, including skin fibroblasts. To gain some understanding on the molecular basis of HF, we characterized iron metabolism in primary cultures of human skin fibroblasts from an individual with the FTL c.497_498dupTC mutation.
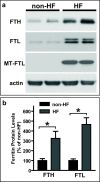
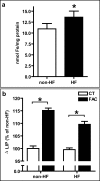
Results: Compared to normal controls, HF fibroblasts showed abnormal iron metabolism consisting of increased levels of ferritin polypeptides, divalent metal transporter 1, basal iron content and reactive oxygen species, and decreased levels of transferrin receptor-1 and IRE-IRP binding activity.
Conclusions: Our data indicates that HF fibroblasts replicate the abnormal iron metabolism observed in the CNS of patients with HF. We propose that HF fibroblasts are a unique cellular model in which to study the role of abnormal iron metabolism in the pathogenesis of HF without artifacts derived from over-expression or lack of endogenous translational regulatory elements.
Figures

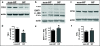



Similar articles
-
Iron, Ferritin, Hereditary Ferritinopathy, and Neurodegeneration.Front Neurosci. 2019 Dec 11;13:1195. doi: 10.3389/fnins.2019.01195. eCollection 2019. Front Neurosci. 2019. PMID: 31920471 Free PMC article. Review.
-
Neuropathological and biochemical investigation of Hereditary Ferritinopathy cases with ferritin light chain mutation: Prominent protein aggregation in the absence of major mitochondrial or oxidative stress.Neuropathol Appl Neurobiol. 2021 Feb;47(1):26-42. doi: 10.1111/nan.12634. Epub 2020 Jun 19. Neuropathol Appl Neurobiol. 2021. PMID: 32464705
-
Expression of a mutant form of the ferritin light chain gene induces neurodegeneration and iron overload in transgenic mice.J Neurosci. 2008 Jan 2;28(1):60-7. doi: 10.1523/JNEUROSCI.3962-07.2008. J Neurosci. 2008. PMID: 18171923 Free PMC article.
-
Effect of Systemic Iron Overload and a Chelation Therapy in a Mouse Model of the Neurodegenerative Disease Hereditary Ferritinopathy.PLoS One. 2016 Aug 30;11(8):e0161341. doi: 10.1371/journal.pone.0161341. eCollection 2016. PLoS One. 2016. PMID: 27574973 Free PMC article.
-
Rare causes of hereditary iron overload.Semin Hematol. 2002 Oct;39(4):249-62. doi: 10.1053/shem.2002.35638. Semin Hematol. 2002. PMID: 12382200 Review.
Cited by
-
Nrf2 activators for the treatment of rare iron overload diseases: From bench to bedside.Redox Biol. 2025 Apr;81:103551. doi: 10.1016/j.redox.2025.103551. Epub 2025 Feb 14. Redox Biol. 2025. PMID: 39965404 Free PMC article. Review.
-
Exploratory Analysis of Image-Guided Ionizing Radiation Delivery to Induce Long-Term Iron Accumulation and Ferritin Expression in a Lung Injury Model: Preliminary Results.Bioengineering (Basel). 2024 Feb 14;11(2):182. doi: 10.3390/bioengineering11020182. Bioengineering (Basel). 2024. PMID: 38391668 Free PMC article.
-
Iron, Ferritin, Hereditary Ferritinopathy, and Neurodegeneration.Front Neurosci. 2019 Dec 11;13:1195. doi: 10.3389/fnins.2019.01195. eCollection 2019. Front Neurosci. 2019. PMID: 31920471 Free PMC article. Review.
-
FLCN regulates transferrin receptor 1 transport and iron homeostasis.J Biol Chem. 2021 Jan-Jun;296:100426. doi: 10.1016/j.jbc.2021.100426. Epub 2021 Feb 17. J Biol Chem. 2021. PMID: 33609526 Free PMC article.
-
Neurodegeneration with brain iron accumulation: update on pathogenic mechanisms.Front Pharmacol. 2014 May 7;5:99. doi: 10.3389/fphar.2014.00099. eCollection 2014. Front Pharmacol. 2014. PMID: 24847269 Free PMC article. Review.
References
-
- Berg D, Youdim MB. Role of iron in neurodegenerative disorders. Top Magn Reson Imaging. 2006;17:5–17. doi: 10.1097/01.rmr.0000245461.90406.ad. - DOI - PubMed
-
- Vidal R, Delisle MB, Ghetti B. Neurodegeneration caused by proteins with an aberrant carboxyl-terminus. J Neuropathol Exp Neurol. 2004;63:787–800. - PubMed
-
- Curtis AR, Fey C, Morris CM, Bindoff LA, Ince PG, Chinnery PF, Coulthard A, Jackson MJ, Jackson AP, McHale DP, Hay D, Barker WA, Markham AF, Bates D, Curtis A, Burn J. Mutation in the gene encoding ferritin light polypeptide causes dominant adult-onset basal ganglia disease. Nat Genet. 2001;28:350–354. doi: 10.1038/ng571. - DOI - PubMed
-
- Vidal R, Ghetti B, Takao M, Brefel-Courbon C, Uro-Coste E, Glazier BS, Siani V, Benson MD, Calvas P, Miravalle L, Rascol O, Delisle MB. Intracellular ferritin accumulation in neural and extraneural tissue characterizes a neurodegenerative disease associated with a mutation in the ferritin light polypeptide gene. J Neuropathol Exp Neurol. 2004;63:363–380. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

