Prevention of NKT cell activation accelerates cutaneous wound closure and alters local inflammatory signals
- PMID: 21067780
- PMCID: PMC3324976
- DOI: 10.1016/j.jss.2010.03.030
Prevention of NKT cell activation accelerates cutaneous wound closure and alters local inflammatory signals
Abstract
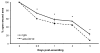
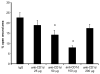
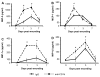
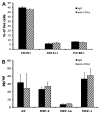
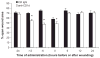
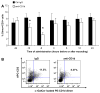
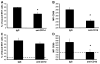
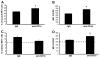
We previously reported that in the absence of NKT cells, wound closure was accelerated in a murine excisional punch wound model. Here, we explored whether purposefully inhibiting NKT cell activation had similar effects on wound closure and the dermal inflammatory response to injury. We found that prevention of NKT cell activation accelerated wound closure in a dose-responsive manner. If anti-CD1d was administered before wounding, NKT cell infiltration into cutaneous wounds was diminished without quantitative changes in cellular infiltrates. Furthermore, prevention of NKT cell activation transiently enhanced the local production of a subset of chemokines, including MIP-2, MCP-1, MIP-1α, and MIP-1β, and altered the relative expression of CD69 and CXCR2 on the surface of both circulating and wound NKT cells. Taken together, these findings suggest that wounding activates NKT cells via CD1d presentation of glycolipid antigen and help further define a role for NKT cells in the regulation of wound inflammation and closure. Many soluble factors have been targeted as potential wound healing therapies, but their clinical success has been limited. Given our findings, the NKT cell may be an attractive target for wound healing therapies.
Copyright © 2011. Published by Elsevier Inc.
Figures

Similar articles
-
A novel role for NKT cells in cutaneous wound repair.J Surg Res. 2011 Jun 15;168(2):325-33.e1. doi: 10.1016/j.jss.2009.09.030. Epub 2009 Oct 9. J Surg Res. 2011. PMID: 20089261 Free PMC article.
-
The Role of Adaptor Proteins in the Biology of Natural Killer T (NKT) Cells.Front Immunol. 2019 Jun 25;10:1449. doi: 10.3389/fimmu.2019.01449. eCollection 2019. Front Immunol. 2019. PMID: 31293596 Free PMC article. Review.
-
UVB-induced skin inflammation and cutaneous tissue injury is dependent on the MHC class I-like protein, CD1d.J Invest Dermatol. 2014 Jan;134(1):192-202. doi: 10.1038/jid.2013.300. Epub 2013 Jul 18. J Invest Dermatol. 2014. PMID: 23867896 Free PMC article.
-
Invariant NKT cells promote skin wound healing by preventing a prolonged neutrophilic inflammatory response.Wound Repair Regen. 2017 Sep;25(5):805-815. doi: 10.1111/wrr.12588. Epub 2017 Dec 8. Wound Repair Regen. 2017. PMID: 28940971
-
CXC chemokines and their receptors: a case for a significant biological role in cutaneous wound healing.Histol Histopathol. 2008 Nov;23(11):1399-407. doi: 10.14670/HH-23.1399. Histol Histopathol. 2008. PMID: 18785122 Free PMC article. Review.
Cited by
-
G-CSF enhances resolution of Staphylococcus aureus wound infection in an age-dependent manner.Shock. 2013 Oct;40(4):327-33. doi: 10.1097/SHK.0b013e3182a43651. Shock. 2013. PMID: 23856924 Free PMC article.
-
Early-life imprinting of unconventional T cells and tissue homeostasis.Science. 2021 Dec 10;374(6573):eabf0095. doi: 10.1126/science.abf0095. Epub 2021 Dec 10. Science. 2021. PMID: 34882451 Free PMC article. Review.
-
Chemokines in health and disease.Exp Cell Res. 2011 Mar 10;317(5):575-89. doi: 10.1016/j.yexcr.2011.01.005. Epub 2011 Jan 9. Exp Cell Res. 2011. PMID: 21223965 Free PMC article. Review.
-
Cutaneous Wound Healing: A Review about Innate Immune Response and Current Therapeutic Applications.Mediators Inflamm. 2022 Apr 25;2022:5344085. doi: 10.1155/2022/5344085. eCollection 2022. Mediators Inflamm. 2022. PMID: 35509434 Free PMC article. Review.
-
Machine learning links different gene patterns of viral infection to immunosuppression and immune-related biomarkers in severe burns.Front Immunol. 2022 Nov 28;13:1054407. doi: 10.3389/fimmu.2022.1054407. eCollection 2022. Front Immunol. 2022. PMID: 36518755 Free PMC article.
References
-
- Petrie NC, Yao F, Eriksson E. Gene therapy in wound healing. Surg Clin N Am. 2003;83:597. - PubMed
-
- Frykberg RG, Armstrong DG, Giurini J, et al. Diabetic foot disorders: A clinical practice guideline. American college of foot and ankle surgeons. J Foot Ankle Surg. 2000;39:S1. - PubMed
-
- Harrington C, Zagari MJ, Corea J, et al. A cost analysis of diabetic lower-extremity ulcers. Diabetes Care. 2000;23:1333. - PubMed
-
- Bendelac A, Lantz O, Quimby ME, et al. CD1 recognition by mouse NK+ T lymphocytes. Science. 1995;268:863. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

