Pnpla3/Adiponutrin deficiency in mice does not contribute to fatty liver disease or metabolic syndrome
- PMID: 21068004
- PMCID: PMC3023552
- DOI: 10.1194/jlr.M011205
Pnpla3/Adiponutrin deficiency in mice does not contribute to fatty liver disease or metabolic syndrome
Abstract
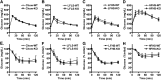
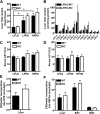
PNPLA3 (adiponutrin, calcium-independent phospholipase A(2) epsilon [iPLA(2)ε]) is an adipose-enriched, nutritionally regulated protein that belongs to the patatin-like phospholipase domain containing (PNPLA) family of lipid metabolizing proteins. Genetic variations in the human PNPLA3 gene (i.e., the rs738409 I148M allele) has been strongly and repeatedly associated with fatty liver disease. Although human PNPLA3 has triacylglycerol (TAG) hydrolase and transacylase activities in vitro, its in vivo function and physiological relevance remain controversial. The objective of this study was to determine the metabolic consequences of global targeted deletion of the Pnpla3 gene in mice. We found that Pnpla3 mRNA expression is altered in adipose tissue and liver in response to acute and chronic nutritional challenges. However, global targeted deletion of the Pnpla3 gene in mice did not affect TAG hydrolysis, nor did it influence energy/glucose/lipid homoeostasis or hepatic steatosis/injury. Experimental interventions designed to increase Pnpla3 expression (refeeding, high-sucrose diet, diet-induced obesity, and liver X receptor agonism) likewise failed to reveal differences in the above-mentioned metabolic phenotypes. Expression of the Pnpla3 paralog, Pnpla5, was increased in adipose tissue but not in liver of Pnpla3-deficient mice, but compensatory regulation of genes involved in TAG metabolism was not identified. Together these data argue against a role for Pnpla3 loss-of-function in fatty liver disease or metabolic syndrome in mice.
Figures

Similar articles
-
Patatin-like phospholipase domain-containing 3/adiponutrin deficiency in mice is not associated with fatty liver disease.Hepatology. 2010 Sep;52(3):1134-42. doi: 10.1002/hep.23812. Hepatology. 2010. PMID: 20648554 Free PMC article.
-
Chronic overexpression of PNPLA3I148M in mouse liver causes hepatic steatosis.J Clin Invest. 2012 Nov;122(11):4130-44. doi: 10.1172/JCI65179. J Clin Invest. 2012. PMID: 23023705 Free PMC article.
-
Shifts in dietary carbohydrate-lipid exposure regulate expression of the non-alcoholic fatty liver disease-associated gene PNPLA3/adiponutrin in mouse liver and HepG2 human liver cells.Metabolism. 2014 Oct;63(10):1352-62. doi: 10.1016/j.metabol.2014.06.016. Epub 2014 Jun 21. Metabolism. 2014. PMID: 25060692 Free PMC article.
-
Unravelling the pathogenesis of fatty liver disease: patatin-like phospholipase domain-containing 3 protein.Curr Opin Lipidol. 2010 Jun;21(3):247-52. doi: 10.1097/mol.0b013e328338ca61. Curr Opin Lipidol. 2010. PMID: 20480550 Review.
-
Function of PNPLA3 I148M-Lessons From In Vivo Studies in Humans.Liver Int. 2025 Apr;45(4):e70047. doi: 10.1111/liv.70047. Liver Int. 2025. PMID: 40052746 Review.
Cited by
-
Lipid droplets and cellular lipid metabolism.Annu Rev Biochem. 2012;81:687-714. doi: 10.1146/annurev-biochem-061009-102430. Epub 2012 Apr 13. Annu Rev Biochem. 2012. PMID: 22524315 Free PMC article. Review.
-
Playing Jekyll and Hyde-The Dual Role of Lipids in Fatty Liver Disease.Cells. 2020 Oct 6;9(10):2244. doi: 10.3390/cells9102244. Cells. 2020. PMID: 33036257 Free PMC article. Review.
-
PNPLA3 I148M mediates the regulatory effect of NF-kB on inflammation in PA-treated HepG2 cells.J Cell Mol Med. 2020 Jan;24(2):1541-1552. doi: 10.1111/jcmm.14839. Epub 2019 Dec 3. J Cell Mol Med. 2020. PMID: 31793207 Free PMC article.
-
Hepatic patatin-like phospholipase domain-containing 3 levels are increased in I148M risk allele carriers and correlate with NAFLD in humans.Hepatol Commun. 2022 Oct;6(10):2689-2701. doi: 10.1002/hep4.2032. Epub 2022 Jul 14. Hepatol Commun. 2022. PMID: 35833455 Free PMC article.
-
Patatin-like phospholipase domain–containing protein 3 promotes transfer of essential fatty acids from triglycerides to phospholipids in hepatic lipid droplets.J Biol Chem. 2018 May 4;293(18):6958-6968. doi: 10.1074/jbc.RA118.002333. Epub 2018 Mar 19. J Biol Chem. 2018. PMID: 29555681 Free PMC article.
References
-
- Schaffer J. E. 2003. Lipotoxicity: when tissues overeat. Curr. Opin. Lipidol. 14: 281–287. - PubMed
-
- Unger R. H. 2003. Minireview: weapons of lean body mass destruction: the role of ectopic lipids in the metabolic syndrome. Endocrinology. 144: 5159–5165. - PubMed
-
- Stefan N., Kantartzis K., Haring H. U. 2008. Causes and metabolic consequences of Fatty liver. Endocr. Rev. 29: 939–960. - PubMed
-
- Marchesini G., Brizi M., Bianchi G., Tomassetti S., Bugianesi E., Lenzi M., McCullough A. J., Natale S., Forlani G., Melchionda N. 2001. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 50: 1844–1850. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F 3001/FWF_/Austrian Science Fund FWF/Austria
- P01 HL020948/HL/NHLBI NIH HHS/United States
- P01 HL-020948/HL/NHLBI NIH HHS/United States
- Z 136/FWF_/Austrian Science Fund FWF/Austria
- HHMI/Howard Hughes Medical Institute/United States
- F 3002/FWF_/Austrian Science Fund FWF/Austria
- P01 HL-057278/HL/NHLBI NIH HHS/United States
- RL1 HL-092550/HL/NHLBI NIH HHS/United States
- P30 DK036836/DK/NIDDK NIH HHS/United States
- RL1 HL092550/HL/NHLBI NIH HHS/United States
- P30-DK-036836/DK/NIDDK NIH HHS/United States
- P01 HL057278/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

