Glyceroneogenesis is inhibited through HIV protease inhibitor-induced inflammation in human subcutaneous but not visceral adipose tissue
- PMID: 21068005
- PMCID: PMC3023541
- DOI: 10.1194/jlr.M000869
Glyceroneogenesis is inhibited through HIV protease inhibitor-induced inflammation in human subcutaneous but not visceral adipose tissue
Abstract
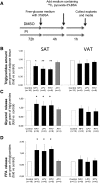
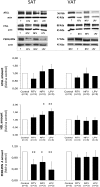
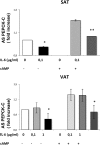
Glyceroneogenesis, a metabolic pathway that participates during lipolysis in the recycling of free fatty acids to triglycerides into adipocytes, contributes to the lipid-buffering function of adipose tissue. We investigated whether glyceroneogenesis could be affected by human immunodeficiency virus (HIV) protease inhibitors (PIs) responsible or not for dyslipidemia in HIV-infected patients. We treated explants obtained from subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) depots from lean individuals. We observed that the dyslipidemic PIs nelfinavir, lopinavir and ritonavir, but not the lipid-neutral PI atazanavir, increased lipolysis and decreased glyceroneogenesis, leading to an increased release of fatty acids from SAT but not from VAT. At the same time, dyslipidemic PIs decreased the amount of perilipin and increased interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) secretion in SAT but not in VAT. Parthenolide, an inhibitor of the NFκB pathway, counteracted PI-induced increased inflammation and decreased glyceroneogenesis. IL-6 (100 ng) inhibited the activity of phosphoenolpyruvate carboxykinase, the key enzyme of glyceroneogenesis, in SAT but not in VAT. Our data show that dyslipidemic but not lipid-neutral PIs decreased glyceroneogenesis as a consequence of PI-induced increased inflammation in SAT that could have an affect on adipocytes and/or macrophages. These results add a new link between fat inflammation and increased fatty acids release and suggest a greater sensitivity of SAT than VAT to PI-induced inflammation.
Figures




Similar articles
-
Visceral and subcutaneous adipose tissue from lean women respond differently to lipopolysaccharide-induced alteration of inflammation and glyceroneogenesis.Nutr Diabetes. 2012 Dec 3;2(12):e51. doi: 10.1038/nutd.2012.29. Nutr Diabetes. 2012. PMID: 23208412 Free PMC article.
-
Impact of skeletal muscle IL-6 on subcutaneous and visceral adipose tissue metabolism immediately after high- and moderate-intensity exercises.Pflugers Arch. 2020 Feb;472(2):217-233. doi: 10.1007/s00424-019-02332-w. Epub 2019 Nov 28. Pflugers Arch. 2020. PMID: 31781893
-
GS-8374, a novel HIV protease inhibitor, does not alter glucose homeostasis in cultured adipocytes or in a healthy-rodent model system.Antimicrob Agents Chemother. 2011 Apr;55(4):1377-82. doi: 10.1128/AAC.01184-10. Epub 2011 Jan 18. Antimicrob Agents Chemother. 2011. PMID: 21245443 Free PMC article.
-
Lopinavir/ritonavir: a review of its use in the management of HIV infection.Drugs. 2006;66(9):1275-99. doi: 10.2165/00003495-200666090-00012. Drugs. 2006. PMID: 16827606 Review.
-
Two Faces of White Adipose Tissue with Heterogeneous Adipogenic Progenitors.Diabetes Metab J. 2019 Dec;43(6):752-762. doi: 10.4093/dmj.2019.0174. Diabetes Metab J. 2019. PMID: 31902145 Free PMC article. Review.
Cited by
-
Adipose Tissue in HIV Infection.Compr Physiol. 2017 Sep 12;7(4):1339-1357. doi: 10.1002/cphy.c160028. Compr Physiol. 2017. PMID: 28915327 Free PMC article. Review.
-
HIV and antiretroviral therapy-related fat alterations.Nat Rev Dis Primers. 2020 Jun 18;6(1):48. doi: 10.1038/s41572-020-0181-1. Nat Rev Dis Primers. 2020. PMID: 32555389 Review.
-
Weight Gain and Antiretroviral Therapy.Infect Dis Clin North Am. 2024 Sep;38(3):499-515. doi: 10.1016/j.idc.2024.04.005. Epub 2024 Jun 12. Infect Dis Clin North Am. 2024. PMID: 38871568 Free PMC article. Review.
-
IL-6 indirectly modulates the induction of glyceroneogenic enzymes in adipose tissue during exercise.PLoS One. 2012;7(7):e41719. doi: 10.1371/journal.pone.0041719. Epub 2012 Jul 23. PLoS One. 2012. PMID: 22844518 Free PMC article.
-
Visceral and subcutaneous adipose tissue from lean women respond differently to lipopolysaccharide-induced alteration of inflammation and glyceroneogenesis.Nutr Diabetes. 2012 Dec 3;2(12):e51. doi: 10.1038/nutd.2012.29. Nutr Diabetes. 2012. PMID: 23208412 Free PMC article.
References
-
- Sevastianova K., Sutinen J., Kannisto K., Hamsten A., Ristola M., Yvi-järvinen H. 2008. Adipose tissue inflammation and liver fat in patients with highly active antiretroviral therapy-associated lipodystrophy. Am. J. Physiol. Endocrinol. Metab. 295: E85–E91. - PubMed
-
- Gan S. K., Samaras K., Thompson C. H., Kraegen E. W., Carr A., Cooper D. A., Chisholm D. J. 2002. Altered myocellular and abdominal fat partioning predict disturbance in insulin action in HIV protease inhibitor-related lipodystrophy. Diabetes. 51: 3163–3169. - PubMed
-
- Leroyer S. N., Tordjman J., Chauvet G., Quette J., Chapron C., Forest C., Antoine B. 2006. Rosiglitazone controls fatty acid cycling in human adipose tissue by means of glyceroneogenesis and glycerol phosphorylation. J. Biol. Chem. 281: 13141–13149. - PubMed
-
- Tordjman J., Chauvet G., Quette J., Beale E. G., Forest C., Antoine B. 2003. Thiazolidinediones block fatty acid release by inducing glyceroneogenesis in fat cells. J. Biol. Chem. 278: 18785–18790. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

