Parathyroid hormone-like hormone (PTHLH) represses decidualization of human uterine fibroblast cells by an autocrine/paracrine mechanism
- PMID: 21068146
- PMCID: PMC3048318
- DOI: 10.1210/jc.2010-1790
Parathyroid hormone-like hormone (PTHLH) represses decidualization of human uterine fibroblast cells by an autocrine/paracrine mechanism
Abstract
Context: Parathyroid hormone-like hormone (PTHLH) is abundantly expressed by human endometrial stromal cells during decidualization. However, the role for PTHLH in the decidualization process is unknown.
Objective: To examine the effects of PTHLH on the induction and maintenance of decidualization of human uterine fibroblast (HUF) cells in vitro.
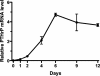
Design: HUF cells were treated with a PTHLH siRNA or a PTHLH receptor antagonist (bPTH(7-34)) before or after decidualization with medroxyprogesterone acetate (MPA), estradiol (E(2)), and prostaglandin E(2) (PGE(2)). Decidualization was monitored by immunocytochemistry and the induction of decidualization-specific marker genes, including IGFBP-1, prolactin, lefty, and transcription factor FOXO1.
Results: HUF cells decidualized after pretreatment with a PTHLH siRNA showed greater morphologic changes of decidualization, greater IGFBP-1 protein, and two- to threefold more IGFBP-1, prolactin, lefty, and FOXO1 mRNAs than cells pretreated with a nonsilencing RNA. The PTHLH siRNA pretreated cells also had 31% less DNA fragmentation (TUNEL assay) and 30-35% less caspase 3 levels during decidualization than cells pretreated treated with nonsilencing RNA. Treatment of HUF cells with PTHLH siRNA or bPTH(7-34) at 9 d after the induction of decidualization also resulted in 2.1- to 3.2-fold greater IGFBP-1, prolactin, lefty, and FOXO1 mRNA levels than that noted in control cells treated with nonsilencing RNA.
Conclusions: These finding strongly suggest that PTHLH represses the induction of human decidualization, stimulates stromal cell apoptosis, and limits the extent of uterine stromal cell differentiation. Because PTHLH and its receptor are expressed by HUF cells and placental cells, the inhibitory effect of PTHLH on decidualization appears to be due, at least in part, to an autocrine/paracrine mechanism.
Figures


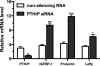
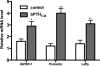

Similar articles
-
Critical role for TWIST1 in the induction of human uterine decidualization.Endocrinology. 2011 Nov;152(11):4368-76. doi: 10.1210/en.2011-1140. Epub 2011 Sep 13. Endocrinology. 2011. PMID: 21914771 Free PMC article.
-
Autocrine prolactin inhibits human uterine decidualization: a novel role for prolactin.Biol Reprod. 2007 May;76(5):777-83. doi: 10.1095/biolreprod.106.053058. Epub 2007 Jan 31. Biol Reprod. 2007. PMID: 17267700
-
LEFTY, a member of the transforming growth factor-beta superfamily, inhibits uterine stromal cell differentiation: a novel autocrine role.Endocrinology. 2010 Mar;151(3):1320-30. doi: 10.1210/en.2009-1081. Epub 2010 Jan 7. Endocrinology. 2010. PMID: 20056823 Free PMC article.
-
Cannabinoid receptor I activation markedly inhibits human decidualization.Mol Cell Endocrinol. 2005 Jan 14;229(1-2):65-74. doi: 10.1016/j.mce.2004.09.007. Mol Cell Endocrinol. 2005. PMID: 15607530
-
IL-1beta during in vitro decidualization in primate.J Reprod Immunol. 2002 May-Jun;55(1-2):35-47. doi: 10.1016/s0165-0378(01)00141-3. J Reprod Immunol. 2002. PMID: 12062820 Review.
Cited by
-
miRNAs in decidual NK cells: regulators worthy of attention during pregnancy.Reprod Biol Endocrinol. 2021 Oct 2;19(1):150. doi: 10.1186/s12958-021-00812-2. Reprod Biol Endocrinol. 2021. PMID: 34600537 Free PMC article. Review.
-
The Long-Term Cure of Patients With Hereditary Medullary Thyroid Carcinoma: 40 Years of Follow-Up in a Single Center.Dtsch Arztebl Int. 2024 Oct 4;121(20):657-664. doi: 10.3238/arztebl.m2024.0174. Dtsch Arztebl Int. 2024. PMID: 39285761 Free PMC article.
-
Spatiotemporal endometrial transcriptome analysis revealed the luminal epithelium as key player during initial maternal recognition of pregnancy in the mare.Sci Rep. 2021 Nov 16;11(1):22293. doi: 10.1038/s41598-021-01785-3. Sci Rep. 2021. PMID: 34785745 Free PMC article.
-
DLC1-dependent parathyroid hormone-like hormone inhibition suppresses breast cancer bone metastasis.J Clin Invest. 2014 Apr;124(4):1646-59. doi: 10.1172/JCI71812. Epub 2014 Mar 3. J Clin Invest. 2014. PMID: 24590291 Free PMC article.
-
Pregnancy success in mice requires appropriate cannabinoid receptor signaling for primary decidua formation.Elife. 2020 Sep 29;9:e61762. doi: 10.7554/eLife.61762. Elife. 2020. PMID: 32990600 Free PMC article.
References
-
- Strewler GJ. 2000. The physiology of parathyroid hormone-related protein. N Engl J Med 342:177–185 - PubMed
-
- Beck F, Tucci J, Senior PV. 1993. Expression of parathyroid hormone-related protein mRNA by uterine tissues and extraembryonic membranes during gestation in rats. J Reprod Fertil 99:343–352 - PubMed
-
- Williams ED, Major BJ, Martin TJ, Moseley JM, Leaver DD. 1998. Effect of antagonism of the parathyroid hormone (PTH)/PTH-related protein receptor on decidualization in rat uterus. J Reprod Fertil 112:59–67 - PubMed
-
- Brar AK, Handwerger S, Kessler CA, Aronow BJ. 2001. Gene induction and categorical reprogramming during in vitro human endometrial fibroblast decidualization. Physiol Genomics 7:135–148 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

