Development of inhibitory timescales in auditory cortex
- PMID: 21068186
- PMCID: PMC3097987
- DOI: 10.1093/cercor/bhq214
Development of inhibitory timescales in auditory cortex
Abstract
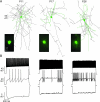
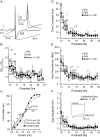
The time course of inhibition plays an important role in cortical sensitivity, tuning, and temporal response properties. We investigated the development of L2/3 inhibitory circuitry between fast-spiking (FS) interneurons and pyramidal cells (PCs) in auditory thalamocortical slices from mice between postnatal day 10 (P10) and P29. We found that the maturation of the intrinsic and synaptic properties of both FS cells and their connected PCs influence the timescales of inhibition. FS cell firing rates increased with age owing to decreased membrane time constants, shorter afterhyperpolarizations, and narrower action potentials. Between FS-PC pairs, excitatory postsynaptic potentials (EPSPs) and inhibitory postsynaptic potentials (IPSPs) changed with age. The latencies, rise, and peak times of the IPSPs, as well as the decay constants of both EPSPs and IPSPs decreased between P10 and P29. In addition, decreases in short-term depression at excitatory PC-FS synapses resulted in more sustained synaptic responses during repetitive stimulation. Finally, we show that during early development, the temporal properties that influence the recruitment of inhibition lag those of excitation. Taken together, our results suggest that the changes in the timescales of inhibitory recruitment coincide with the development of the tuning and temporal response properties of auditory cortical networks.
Figures




Similar articles
-
Cell type-specific, presynaptic LTP of inhibitory synapses on fast-spiking GABAergic neurons in the mouse visual cortex.J Neurosci. 2012 Sep 19;32(38):13189-99. doi: 10.1523/JNEUROSCI.1386-12.2012. J Neurosci. 2012. PMID: 22993435 Free PMC article.
-
Spatial profile of excitatory and inhibitory synaptic connectivity in mouse primary auditory cortex.J Neurosci. 2012 Apr 18;32(16):5609-19. doi: 10.1523/JNEUROSCI.5158-11.2012. J Neurosci. 2012. PMID: 22514322 Free PMC article.
-
Transcriptional and electrophysiological maturation of neocortical fast-spiking GABAergic interneurons.J Neurosci. 2009 May 27;29(21):7040-52. doi: 10.1523/JNEUROSCI.0105-09.2009. J Neurosci. 2009. PMID: 19474331 Free PMC article.
-
Maturation of intrinsic and synaptic properties of layer 2/3 pyramidal neurons in mouse auditory cortex.J Neurophysiol. 2008 Jun;99(6):2998-3008. doi: 10.1152/jn.01160.2007. Epub 2008 Apr 16. J Neurophysiol. 2008. PMID: 18417631 Free PMC article.
-
Short-term plasticity of unitary inhibitory-to-inhibitory synapses depends on the presynaptic interneuron subtype.J Neurosci. 2012 Jan 18;32(3):983-8. doi: 10.1523/JNEUROSCI.5007-11.2012. J Neurosci. 2012. PMID: 22262896 Free PMC article.
Cited by
-
Hearing loss differentially affects thalamic drive to two cortical interneuron subtypes.J Neurophysiol. 2013 Aug;110(4):999-1008. doi: 10.1152/jn.00182.2013. Epub 2013 May 29. J Neurophysiol. 2013. PMID: 23719211 Free PMC article.
-
Rescue of inhibitory synapse strength following developmental hearing loss.PLoS One. 2013;8(1):e53438. doi: 10.1371/journal.pone.0053438. Epub 2013 Jan 11. PLoS One. 2013. PMID: 23326429 Free PMC article.
-
Electrophysiology and morphology of human cortical supragranular pyramidal cells in a wide age range.Elife. 2025 Mar 28;13:RP100390. doi: 10.7554/eLife.100390. Elife. 2025. PMID: 40152903 Free PMC article.
-
Adenosine A1 Receptor mRNA Expression by Neurons and Glia in the Auditory Forebrain.Anat Rec (Hoboken). 2018 Nov;301(11):1882-1905. doi: 10.1002/ar.23907. Epub 2018 Oct 12. Anat Rec (Hoboken). 2018. PMID: 30315630 Free PMC article.
-
Coexistence of lateral and co-tuned inhibitory configurations in cortical networks.PLoS Comput Biol. 2011 Oct;7(10):e1002161. doi: 10.1371/journal.pcbi.1002161. Epub 2011 Oct 6. PLoS Comput Biol. 2011. PMID: 21998561 Free PMC article.
References
-
- Barth DS, MacDonald KD. Thalamic modulation of high-frequency oscillating potentials in auditory cortex. Nature. 1996;383:78–81. - PubMed
-
- Beierlein M, Gibson JR, Connors BW. Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J Neurophysiol. 2003;90:2987–3000. - PubMed
-
- Brosch M, Budinger E, Scheich H. Stimulus-related gamma oscillations in primate auditory cortex. J Neurophysiol. 2002;87:2715–2725. - PubMed

