Human CYP2S1 metabolizes cyclooxygenase- and lipoxygenase-derived eicosanoids
- PMID: 21068195
- PMCID: PMC3033693
- DOI: 10.1124/dmd.110.035121
Human CYP2S1 metabolizes cyclooxygenase- and lipoxygenase-derived eicosanoids
Abstract
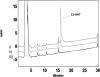
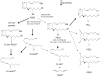
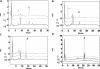
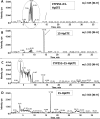
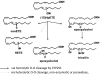
CYP2S1 is a recently described dioxin-inducible cytochrome P450. We previously demonstrated that human CYP2S1 oxidizes a number of carcinogens but only via the peroxide shunt. In this article, we investigated whether human CYP2S1 can metabolize cyclooxygenase- and lipoxygenase-derived lipid peroxides in a NADPH-independent fashion. Human CYP2S1 metabolizes prostaglandin G(2) (PGG(2)) (K(m) = 0.267 ± 0.072 μM) into several products including 12S-hydroxy-5Z,8E,10E-heptadecatrienoic acid (12-HHT). It also metabolizes prostaglandin H(2) (PGH(2)) (K(m) = 11.7 ± 2.8 μM) into malondialdehyde, 12-HHT, and thromboxane A(2) (TXA(2)). The turnover to 12-HHT by human CYP2S1 (1.59 ± 0.04 min(-1)) is 40-fold higher than that of TXA(2) (0.04 min(-1)). In addition to PGG(2) and PGH(2) metabolism, human CYP2S1 efficiently metabolizes the hydroperoxyeicosatetraenoic acids (5S-, 12S-, and 15S-) and 13S-hydroperoxyoctadecadienoic acid into 5-oxo-eicosatetraenoic acid (turnover = 16.7 ± 0.3 min(-1)), 12-oxo-eicosatetraenoic acid 1 (11.5 ± 0.9 min(-1)), 15-oxo-eicosatetraenoic acid (16.9 ± 0.8 min(-1)), and 13-octadecadienoic acid (20.2 ± 0.9 min(-1)), respectively. Other cytochromes P450 such as CYP1A1, 1A2, 1B1, and 3A4 underwent similar conversions but at slower rates. The fatty acid hydroperoxides were also converted by human CYP2S1 to several epoxyalcohols. Our data indicate that fatty acid endoperoxides and hydroperoxides represent endogenous substrates of CYP2S1 and suggest that the enzyme CYP2S1 may play an important role in the inflammatory process because some of the products that CYP2S1 produces play important roles in inflammation.
Figures





References
-
- Altmann R, Hausmann M, Spöttl T, Gruber M, Bull AW, Menzel K, Vogl D, Herfarth H, Schölmerich J, Falk W, et al. (2007) 13-Oxo-ODE is an endogenous ligand for PPARγ in human colonic epithelial cells. Biochem Pharmacol 74:612–622 - PubMed
-
- Antón R, Camacho M, Puig L, Vila L. (2002) Hepoxilin B3 and its enzymatically formed derivative trioxilin B3 are incorporated into phospholipids in psoriatic lesions. J Invest Dermatol 118:139–146 - PubMed
-
- Barnes PJ, Rodger IW, Thomson NC. (1992) Asthma: Basic Mechanisms and Clinical Management, Academic Press, San Diego
-
- Bièche I, Narjoz C, Asselah T, Vacher S, Marcellin P, Lidereau R, Beaune P, de Waziers I. (2007) Reverse transcriptase-PCR quantification of mRNA levels from cytochrome (CYP)1, CYP2 and CYP3 families in 22 different human tissues. Pharmacogenet Genomics 17:731–742 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

