Pathogen-induced heart rate changes associated with cholinergic nervous system activation
- PMID: 21068197
- PMCID: PMC3043803
- DOI: 10.1152/ajpregu.00487.2010
Pathogen-induced heart rate changes associated with cholinergic nervous system activation
Abstract
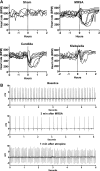
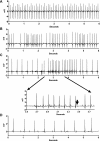
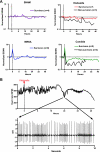
The autonomic nervous system plays a central role in regulation of host defense and in physiological responses to sepsis, including changes in heart rate and heart rate variability. The cholinergic anti-inflammatory response, whereby infection triggers vagal efferent signals that dampen production of proinflammatory cytokines, would be predicted to result in increased vagal signaling to the heart and increased heart rate variability. In fact, decreased heart rate variability is widely described in humans with sepsis. Our studies elucidate this apparent paradox by showing that mice injected with pathogens demonstrate transient bradyarrhythmias of vagal origin in a background of decreased heart rate variability (HRV). Intraperitoneal injection of a large inoculum of Gram-positive or Gram-negative bacteria or Candida albicans rapidly induced bradyarrhythmias of sinus and AV nodal block, characteristic of cardiac vagal firing and dramatically increased short-term HRV. These pathogen-induced bradycardias were immediately terminated by atropine, an antagonist of muscarinic cholinergic receptors, demonstrating the role of vagal efferent signaling in this response. Vagal afferent signaling following pathogen injection was demonstrated by intense nuclear c-Fos activity in neurons of the vagal sensory ganglia and brain stem. Surprisingly, pathogen-induced bradycardia demonstrated rapid and prolonged desensitization and did not recur on repeat injection of the same organism 3 h or 3 days after the initial exposure. After recovery from the initial bradycardia, depressed heart rate variability developed in some mice and was correlated with elevated plasma cytokine levels and mortality. Our findings of decreased HRV and transient heart rate decelerations in infected mice are similar to heart rate changes described by our group in preterm neonates with sepsis. Pathogen sensing and signaling via the vagus nerve, and the desensitization of this response, may account for periods of both increased and decreased heart rate variability in sepsis.
Figures


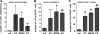

References
-
- American Physiological Society Guiding principles for research involving animals, and human beings. Am J Physiol Regul Integr Comp Physiol 283: R281–R283, 2002 - PubMed
-
- Akiho H, Khan WI, Al-Kaabi A, Blennerhassett P, Deng Y, Collins SM. Cytokine modulation of muscarinic receptors in the murine intestine. Am J Physiol Gastrointest Liver Physiol 293: G250–G255, 2007 - PubMed
-
- Barnes PJ, Haddad EB, Rousell J. Regulation of muscarinic M2 receptors. Life Sci 60: 1015–1021, 1997 - PubMed
-
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405: 458–462, 2000 - PubMed
-
- Cao H, Lake DE, Griffin MP, Moorman JR. Increased nonstationarity of neonatal heart rate before the clinical diagnosis of sepsis. Ann Biomed Eng 32: 233–244, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

