Effect of oral snus and medicinal nicotine in smokers on toxicant exposure and withdrawal symptoms: a feasibility study
- PMID: 21068204
- PMCID: PMC3063713
- DOI: 10.1158/1055-9965.EPI-10-0349
Effect of oral snus and medicinal nicotine in smokers on toxicant exposure and withdrawal symptoms: a feasibility study
Erratum in
- Cancer Epidemiol Biomarkers Prev. 2011 May;20(5):1048
Abstract
Background: Smokeless, spitless tobacco products are being introduced and marketed as cigarette substitutes. Data are needed regarding how smokers interested in cessation would use these products, the levels of resultant toxicant exposure, and the feasibility of using these products as aids for tobacco cessation.
Methods: Smokers were randomized to receive Camel Snus (n = 51), Taboka (n = 52), or medicinal nicotine (n = 27) and required to quit smoking for 4 weeks. Measures of toxicant exposure and symptoms of craving and withdrawal were assessed prior to and during product use.
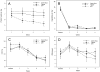
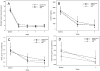
Results: Concentrations of exhaled carbon monoxide, urinary cotinine, urinary 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol and its glucuronides (total NNAL), and urinary N'-nitrosonornicotine and its glucuronide (total NNN) were significantly (P values <0.05) lower at the end of treatment in each group except for total NNN in those receiving Camel Snus (P = 0.066). A significant group × time effect was observed for total NNAL concentrations (P = 0.002) with the decrease greatest in the medicinal nicotine group and smallest decrease in the Camel Snus group. No significant differences between groups were found in craving and withdrawal symptoms.
Conclusions: Enrolling smokers into a cessation study utilizing newer smokeless tobacco products is feasible. Camel Snus and Taboka use was not found to be superior to medicinal nicotine in reducing withdrawal symptoms but decreases in NNAL were smaller in users of Camel Snus.
Impact: This study demonstrates the feasibility of conducting a smoking cessation study utilizing these newer tobacco products. An appropriately powered study is needed to assess smoking cessation rates using these newer products compared with established, safer products such as medicinal nicotine.
Trial registration: ClinicalTrials.gov NCT00469079.
©2011 AACR.
Figures
Similar articles
-
Evaluation of carcinogen exposure in people who used "reduced exposure" tobacco products.J Natl Cancer Inst. 2004 Jun 2;96(11):844-52. doi: 10.1093/jnci/djh163. J Natl Cancer Inst. 2004. PMID: 15173268 Clinical Trial.
-
Randomised clinical trial of snus versus medicinal nicotine among smokers interested in product switching.Tob Control. 2016 May;25(3):267-74. doi: 10.1136/tobaccocontrol-2014-052080. Epub 2015 May 19. Tob Control. 2016. PMID: 25991608 Free PMC article. Clinical Trial.
-
Potential reduced exposure products (PREPs) for smokeless tobacco users: clinical evaluation methodology.Nicotine Tob Res. 2008 Sep;10(9):1441-8. doi: 10.1080/14622200802323258. Nicotine Tob Res. 2008. PMID: 19023835 Free PMC article. Clinical Trial.
-
[Harm reduction strategy in tobacco control].Epidemiol Prev. 2011 May-Aug;35(3-4 Suppl 1):19-32. Epidemiol Prev. 2011. PMID: 21926451 Review. Italian.
-
Nicotine chemistry, metabolism, kinetics and biomarkers.Handb Exp Pharmacol. 2009;(192):29-60. doi: 10.1007/978-3-540-69248-5_2. Handb Exp Pharmacol. 2009. PMID: 19184645 Free PMC article. Review.
Cited by
-
Caregivers' interest in using smokeless tobacco products: Novel methods that may reduce children's exposure to secondhand smoke.J Health Psychol. 2016 Oct;21(10):2306-13. doi: 10.1177/1359105315576347. Epub 2015 Apr 6. J Health Psychol. 2016. PMID: 25845835 Free PMC article. Clinical Trial.
-
Biomarkers of exposure to new and emerging tobacco delivery products.Am J Physiol Lung Cell Mol Physiol. 2017 Sep 1;313(3):L425-L452. doi: 10.1152/ajplung.00343.2016. Epub 2017 May 18. Am J Physiol Lung Cell Mol Physiol. 2017. PMID: 28522563 Free PMC article. Review.
-
Consortium on Methods Evaluating Tobacco: Research Tools to Inform US Food and Drug Administration Regulation of Snus.Nicotine Tob Res. 2018 Sep 25;20(11):1292-1300. doi: 10.1093/ntr/ntx228. Nicotine Tob Res. 2018. PMID: 29059363 Free PMC article. Review.
-
Subjective responses to oral tobacco products: scale validation.Nicotine Tob Res. 2013 Jul;15(7):1259-64. doi: 10.1093/ntr/nts265. Epub 2012 Dec 13. Nicotine Tob Res. 2013. PMID: 23239843 Free PMC article.
-
Tobacco-specific N-nitrosamines and polycyclic aromatic hydrocarbons in cigarettes smoked by the participants of the Shanghai Cohort Study.Int J Cancer. 2016 Sep 15;139(6):1261-9. doi: 10.1002/ijc.30178. Epub 2016 Jun 17. Int J Cancer. 2016. PMID: 27163125 Free PMC article.
References
-
- Russell MA, Jarvis MJ, Feyerabend C. A new age for snuff? Lancet. 1980;1:474–5. - PubMed
-
- Rodu B. An alternative approach to smoking control. Am J Med Sci. 1994;308:32–4. - PubMed
-
- Hatsukami DK, Ebbert JO, Feuer RM, Stepanov I, Hecht SS. Changing smokeless tobacco products new tobacco-delivery systems. Am J Prev Med. 2007;33:S368–78. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

