Norovirus P particle, a novel platform for vaccine development and antibody production
- PMID: 21068235
- PMCID: PMC3020015
- DOI: 10.1128/JVI.01835-10
Norovirus P particle, a novel platform for vaccine development and antibody production
Abstract
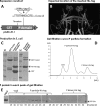
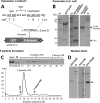
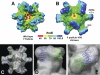
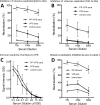
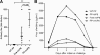
The norovirus P particle is an octahedral nanoparticle formed by 24 copies of the protrusion (P) domain of the norovirus capsid protein. This P particle is easily produced in Escherichia coli, extremely stable, and highly immunogenic. There are three surface loops per P domain, making a total of 72 loops per particle, and these are potential sites for foreign antigen presentation for immune enhancement. To prove this concept, a small peptide (His tag, 7 amino acids [aa]) and a large antigen (rotavirus VP8, 159 aa) were inserted into one of the loops. Neither insertion affects P particle formation, while both antigens were presented well on the P particle surface. The immune-enhancement effect of the P particle was demonstrated by significantly increased antibody titers induced by the P particle-presented antigens compared to the titers induced by free antigens. In addition, the measured neutralization antibody titers and levels of protection against rotavirus shedding in mice immunized with the VP8 chimeric P particles were significantly higher than those of mice immunized with the free VP8 antigen. Sera from P particle-VP8 chimera-vaccinated animals also blocked norovirus virus-like particle (VLP) binding to the histo-blood group antigen (HBGA) receptors. From these data, the P particle appears to be an excellent vaccine platform for antigen presentation. The readily available three surface loops and the great capacity for foreign antigen insertion make this platform attractive for wide application in vaccine development and antibody production. The P particle-VP8 chimeras may serve as a dual vaccine against both rotavirus and norovirus.
Figures
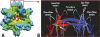




Similar articles
-
Evaluation of a bivalent recombinant vaccine candidate targeting norovirus and rotavirus: Antibodies to rotavirus NSP4 exert antidiarrheal effects without virus neutralization.J Med Virol. 2022 Aug;94(8):3847-3856. doi: 10.1002/jmv.27809. Epub 2022 May 6. J Med Virol. 2022. PMID: 35474320
-
Immune response and protective efficacy of the S particle presented rotavirus VP8* vaccine in mice.Vaccine. 2019 Jul 9;37(30):4103-4110. doi: 10.1016/j.vaccine.2019.05.075. Epub 2019 Jun 11. Vaccine. 2019. PMID: 31201052 Free PMC article.
-
A candidate dual vaccine against influenza and noroviruses.Vaccine. 2011 Oct 13;29(44):7670-7. doi: 10.1016/j.vaccine.2011.07.139. Epub 2011 Aug 11. Vaccine. 2011. PMID: 21839795 Free PMC article.
-
Norovirus P particle: a subviral nanoparticle for vaccine development against norovirus, rotavirus and influenza virus.Nanomedicine (Lond). 2012 Jun;7(6):889-97. doi: 10.2217/nnm.12.62. Nanomedicine (Lond). 2012. PMID: 22734641 Free PMC article. Review.
-
Norovirus Capsid Protein-Derived Nanoparticles and Polymers as Versatile Platforms for Antigen Presentation and Vaccine Development.Pharmaceutics. 2019 Sep 12;11(9):472. doi: 10.3390/pharmaceutics11090472. Pharmaceutics. 2019. PMID: 31547456 Free PMC article. Review.
Cited by
-
Glycan-Induced Protein Dynamics in Human Norovirus P Dimers Depend on Virus Strain and Deamidation Status.Molecules. 2021 Apr 7;26(8):2125. doi: 10.3390/molecules26082125. Molecules. 2021. PMID: 33917179 Free PMC article.
-
Effects of Rotavirus NSP4 on the Immune Response and Protection of Rotavirus-Norovirus Recombinant Subunit Vaccines in Different Immune Pathways.Vaccines (Basel). 2024 Sep 8;12(9):1025. doi: 10.3390/vaccines12091025. Vaccines (Basel). 2024. PMID: 39340055 Free PMC article.
-
Combinations but Not a Single PlpE Epitope Induces Host Protective Immunity against Pasteurella multocida.Infect Immun. 2023 Mar 15;91(3):e0027222. doi: 10.1128/iai.00272-22. Epub 2023 Feb 23. Infect Immun. 2023. PMID: 36815793 Free PMC article.
-
Identifying carbohydrate ligands of a norovirus P particle using a catch and release electrospray ionization mass spectrometry assay.J Am Soc Mass Spectrom. 2014 Jan;25(1):111-9. doi: 10.1007/s13361-013-0752-4. Epub 2013 Oct 5. J Am Soc Mass Spectrom. 2014. PMID: 24096878
-
Norovirus vaccines: Correlates of protection, challenges and limitations.Hum Vaccin Immunother. 2016 Jul 2;12(7):1653-69. doi: 10.1080/21645515.2015.1125054. Epub 2016 Feb 2. Hum Vaccin Immunother. 2016. PMID: 26836766 Free PMC article. Review.
References
-
- Blanchard, H., X. Yu, B. S. Coulson, and M. von Itzstein. 2007. Insight into host cell carbohydrate-recognition by human and porcine rotavirus from crystal structures of the virion spike associated carbohydrate-binding domain (VP8*). J. Mol. Biol. 367:1215-1226. - PubMed
-
- Chatterji, A., L. L. Burns, S. S. Taylor, G. P. Lomonossoff, J. E. Johnson, T. Lin, and C. Porta. 2002. Cowpea mosaic virus: from the presentation of antigenic peptides to the display of active biomaterials. Intervirology 45:362-370. - PubMed
-
- Chatterji, A., W. Ochoa, L. Shamieh, S. P. Salakian, S. M. Wong, G. Clinton, P. Ghosh, T. Lin, and J. E. Johnson. 2004. Chemical conjugation of heterologous proteins on the surface of Cowpea mosaic virus. Bioconjug. Chem. 15:807-813. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

