Oxidative stress induces reactivation of Kaposi's sarcoma-associated herpesvirus and death of primary effusion lymphoma cells
- PMID: 21068240
- PMCID: PMC3020037
- DOI: 10.1128/JVI.01742-10
Oxidative stress induces reactivation of Kaposi's sarcoma-associated herpesvirus and death of primary effusion lymphoma cells
Abstract
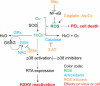
Kaposi's sarcoma (KS) and primary effusion lymphoma (PEL) cells are predominantly infected with latent Kaposi's sarcoma-associated herpesvirus (KSHV), presenting a barrier to the destruction of tumor cells. Latent KSHV can be reactivated to undergo lytic replication. Here we report that in PEL cells, oxidative stress induced by upregulated reactive oxygen species (ROS) can lead to KSHV reactivation or cell death. ROS are upregulated by NF-κB inhibition and are required for subsequent KSHV reactivation. Disruption of the intracellular redox balance through depletion of the antioxidant glutathione or inhibition of the antioxidant enzyme catalase also induces KSHV reactivation, suggesting that hydrogen peroxide induces reactivation. In addition, p38 signaling is required for KSHV reactivation induced by ROS. Furthermore, treatment of PEL cells with a higher concentration of the NF-κB inhibitor than that used for inducing KSHV reactivation further upregulates ROS and induces massive cell death. ROS, but not p38 signaling, are required for PEL cell death induced by NF-κB inhibition as well as by glutathione depletion. Importantly, anticancer drugs, such as cisplatin and arsenic trioxide, also induce KSHV reactivation and PEL cell death in a ROS-dependent manner. Our study thus establishes a critical role for ROS and oxidative stress in the regulation of KSHV reactivation and PEL cell death. Disrupting the cellular redox balance may be a potential strategy for treating KSHV-associated lymphoma.
Figures


Similar articles
-
Reactive oxygen species hydrogen peroxide mediates Kaposi's sarcoma-associated herpesvirus reactivation from latency.PLoS Pathog. 2011 May;7(5):e1002054. doi: 10.1371/journal.ppat.1002054. Epub 2011 May 19. PLoS Pathog. 2011. PMID: 21625536 Free PMC article.
-
Targeting FoxO proteins induces lytic reactivation of KSHV for treating herpesviral primary effusion lymphoma.PLoS Pathog. 2023 Aug 18;19(8):e1011581. doi: 10.1371/journal.ppat.1011581. eCollection 2023 Aug. PLoS Pathog. 2023. PMID: 37594999 Free PMC article.
-
Cdk1 inhibition induces mutually inhibitory apoptosis and reactivation of Kaposi's sarcoma-associated herpesvirus.J Virol. 2012 Jun;86(12):6668-76. doi: 10.1128/JVI.06240-11. Epub 2012 Apr 11. J Virol. 2012. PMID: 22496227 Free PMC article.
-
[Replication Machinery of Kaposi's Sarcoma-associated Herpesvirus and Drug Discovery Research].Yakugaku Zasshi. 2019;139(1):69-73. doi: 10.1248/yakushi.18-00164-2. Yakugaku Zasshi. 2019. PMID: 30606932 Review. Japanese.
-
Regulation of KSHV Latency and Lytic Reactivation.Viruses. 2020 Sep 17;12(9):1034. doi: 10.3390/v12091034. Viruses. 2020. PMID: 32957532 Free PMC article. Review.
Cited by
-
Kaposi's sarcoma-associated herpesvirus induces Nrf2 during de novo infection of endothelial cells to create a microenvironment conducive to infection.PLoS Pathog. 2014 Oct 23;10(10):e1004460. doi: 10.1371/journal.ppat.1004460. eCollection 2014 Oct. PLoS Pathog. 2014. PMID: 25340789 Free PMC article.
-
1, 25(OH)2 D3 Induces Reactivation and Death of Kaposi's Sarcoma-Associated Herpesvirus of Primary Effusion Lymphoma cells.Sci Rep. 2017 Sep 29;7(1):12438. doi: 10.1038/s41598-017-12676-x. Sci Rep. 2017. PMID: 28963501 Free PMC article.
-
Hepatic Kaposi sarcoma: A case report and review of the literature.World J Hepatol. 2017 Feb 8;9(4):171-179. doi: 10.4254/wjh.v9.i4.171. World J Hepatol. 2017. PMID: 28217255 Free PMC article. Review.
-
Oxidative stress induced carbonylation in human plasma.J Proteomics. 2011 Oct 19;74(11):2395-416. doi: 10.1016/j.jprot.2011.07.014. Epub 2011 Jul 30. J Proteomics. 2011. PMID: 21856457 Free PMC article.
-
ATM facilitates mouse gammaherpesvirus reactivation from myeloid cells during chronic infection.Virology. 2015 Sep;483:264-74. doi: 10.1016/j.virol.2015.04.026. Epub 2015 May 21. Virology. 2015. PMID: 26001649 Free PMC article.
References
-
- Ambinder, R. F., K. D. Robertson, S. M. Moore, and J. Yang. 1996. Epstein-Barr virus as a therapeutic target in Hodgkin's disease and nasopharyngeal carcinoma. Semin. Cancer Biol. 7:217-226. - PubMed
-
- Brander, C., T. Suscovich, Y. Lee, P. T. Nguyen, P. O'Connor, J. Seebach, N. G. Jones, M. van Gorder, B. D. Walker, and D. T. Scadden. 2000. Impaired CTL recognition of cells latently infected with Kaposi's sarcoma-associated herpes virus. J. Immunol. 165:2077-2083. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

