Noncytotoxic inhibition of viral infection through eIF4F-independent suppression of translation by 4EGi-1
- PMID: 21068241
- PMCID: PMC3019991
- DOI: 10.1128/JVI.01873-10
Noncytotoxic inhibition of viral infection through eIF4F-independent suppression of translation by 4EGi-1
Abstract
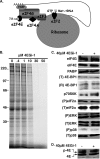
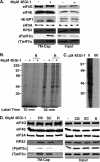
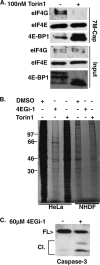
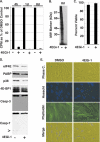
The eukaryotic initiation factor eIF4F recruits ribosomes to capped mRNAs while eIF2 mediates start codon recognition to initiate protein synthesis. Increasing interest in targeting translation to suppress tumor growth has led to the development of new classes of inhibitors, including 4EGi-1, which disrupts eIF4F complexes. However, the full effects of this inhibitor and its potential uses in the treatment of other disease states remain unclear. Here, we show that overall rates of protein synthesis in primary human cells were affected only modestly by eIF4F disruption using the mTOR inhibitor Torin1, yet were highly sensitive to 4EGi-1. Translational suppression occurred even at concentrations of 4EGi-1 that were below those required to significantly alter eIF4F levels but were instead found to increase the association of ribosomal complexes containing inactive eIF2α. Although highly stable in culture, the effects of 4EGi-1 on both cellular protein synthesis and ribosome association were readily reversible upon inhibitor removal. In addition, despite potently inhibiting translation, prolonged exposure to 4EGi-1 had only modest effects on cell morphology and protein abundance without affecting viability or stress tolerance to any significant degree, although differential effects on heat shock protein (hsp) expression highlighted distinct 4EGi-1-sensitive modes of hsp induction. In contrast, 4EGi-1 potently suppressed poxvirus replication as well as both reactivation and lytic phases of herpesvirus infection. These findings identify a novel way in which 4EGi-1 affects the host cell's protein synthesis machinery and demonstrate its potential as a noncytotoxic inhibitor of diverse forms of viral infection.
Figures
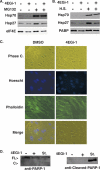
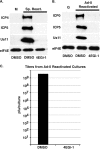
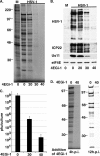
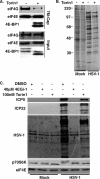
Similar articles
-
Tumor suppression by small molecule inhibitors of translation initiation.Oncotarget. 2012 Aug;3(8):869-81. doi: 10.18632/oncotarget.598. Oncotarget. 2012. PMID: 22935625 Free PMC article.
-
Translation of viral mRNAs that do not require eIF4E is blocked by the inhibitor 4EGI-1.Virology. 2013 Sep;444(1-2):171-80. doi: 10.1016/j.virol.2013.06.008. Epub 2013 Jul 17. Virology. 2013. PMID: 23870416 Free PMC article.
-
The cap-translation inhibitor 4EGI-1 induces apoptosis in multiple myeloma through Noxa induction.Br J Cancer. 2012 May 8;106(10):1660-7. doi: 10.1038/bjc.2012.139. Epub 2012 Apr 17. Br J Cancer. 2012. PMID: 22510748 Free PMC article.
-
Manipulation of the host translation initiation complex eIF4F by DNA viruses.Biochem Soc Trans. 2010 Dec;38(6):1511-6. doi: 10.1042/BST0381511. Biochem Soc Trans. 2010. PMID: 21118117 Review.
-
Regulation of translation initiation by amino acids in eukaryotic cells.Prog Mol Subcell Biol. 2001;26:155-84. doi: 10.1007/978-3-642-56688-2_6. Prog Mol Subcell Biol. 2001. PMID: 11575165 Review.
Cited by
-
High-throughput translational profiling with riboPLATE-seq.Sci Rep. 2022 Apr 5;12(1):5718. doi: 10.1038/s41598-022-09638-3. Sci Rep. 2022. PMID: 35383235 Free PMC article.
-
Consideration of Binding Kinetics in the Design of Stapled Peptide Mimics of the Disordered Proteins Eukaryotic Translation Initiation Factor 4E-Binding Protein 1 and Eukaryotic Translation Initiation Factor 4G.J Med Chem. 2019 May 23;62(10):4967-4978. doi: 10.1021/acs.jmedchem.9b00068. Epub 2019 May 9. J Med Chem. 2019. PMID: 31033289 Free PMC article.
-
The diversity, plasticity, and adaptability of cap-dependent translation initiation and the associated machinery.RNA Biol. 2020 Sep;17(9):1239-1251. doi: 10.1080/15476286.2020.1766179. Epub 2020 Jun 4. RNA Biol. 2020. PMID: 32496897 Free PMC article. Review.
-
Tinkering with translation: protein synthesis in virus-infected cells.Cold Spring Harb Perspect Biol. 2013 Jan 1;5(1):a012351. doi: 10.1101/cshperspect.a012351. Cold Spring Harb Perspect Biol. 2013. PMID: 23209131 Free PMC article. Review.
-
The battle over mTOR: an emerging theatre in host-pathogen immunity.PLoS Pathog. 2012 Sep;8(9):e1002894. doi: 10.1371/journal.ppat.1002894. Epub 2012 Sep 13. PLoS Pathog. 2012. PMID: 23028309 Free PMC article. No abstract available.
References
-
- Beere, H. M., D. B. Wolf, K. Cain, D. D. Mosser, A. Mahboubi, T. Kuwana, P. Tailor, R. I. Morimoto, G. M. Cohen, and D. R. Green. 2000. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat. Cell Biol. 2:469-475. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

