The bovine herpesvirus 4 Bo10 gene encodes a nonessential viral envelope protein that regulates viral tropism through both positive and negative effects
- PMID: 21068242
- PMCID: PMC3019988
- DOI: 10.1128/JVI.01092-10
The bovine herpesvirus 4 Bo10 gene encodes a nonessential viral envelope protein that regulates viral tropism through both positive and negative effects
Abstract
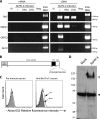
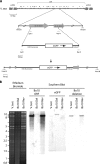
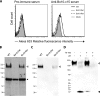
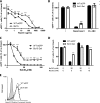
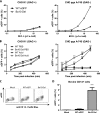
All gammaherpesviruses encode a glycoprotein positionally homologous to the Epstein-Barr virus gp350 and the Kaposi's sarcoma-associated herpesvirus (KSHV) K8.1. In this study, we characterized the positional homologous glycoprotein of bovine herpesvirus 4 (BoHV-4), encoded by the Bo10 gene. We identified a 180-kDa gene product, gp180, that was incorporated into the virion envelope. A Bo10 deletion virus was viable but showed a growth deficit associated with reduced binding to epithelial cells. This seemed to reflect an interaction of gp180 with glycosaminoglycans (GAGs), since compared to the wild-type virus, the Bo10 mutant virus was both less infectious for GAG-positive (GAG(+)) cells and more infectious for GAG-negative (GAG(-)) cells. However, we could not identify a direct interaction between gp180 and GAGs, implying that any direct interaction must be of low affinity. This function of gp180 was very similar to that previously identified for the murid herpesvirus 4 gp150 and also to that of the Epstein-Barr virus gp350 that promotes CD21(+) cell infection and inhibits CD21(-) cell infection. We propose that such proteins generally regulate virion attachment both by binding to cells and by covering another receptor-binding protein until they are displaced. Thus, they regulate viral tropism both positively and negatively depending upon the presence or absence of their receptor.
Figures




References
-
- Baeuerle, P. A., and W. B. Huttner. 1986. Chlorate-a potent inhibitor of protein sulfation in intact cells. Biochem. Biophys. Res. Commun. 141:870-877. - PubMed
-
- Boerner, B., W. Weigelt, H. J. Buhk, G. Castrucci, and H. Ludwig. 1999. A sensitive and specific PCR/Southern blot assay for detection of bovine herpesvirus 4 in calves infected experimentally. J. Virol. Methods 83:169-180. - PubMed
-
- Borza, C. M., and L. M. Hutt-Fletcher. 2002. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nat. Med. 8:594-599. - PubMed
-
- Bublot, M., M. F. Van Bressem, E. Thiry, J. Dubuisson, and P. P. Pastoret. 1990. Bovine herpesvirus 4 genome: cloning, mapping and strain variation analysis. J. Gen. Virol. 71:133-142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

