Prevalence and characterization of human Shigella infections in Henan Province, China, in 2006
- PMID: 21068291
- PMCID: PMC3020427
- DOI: 10.1128/JCM.01508-10
Prevalence and characterization of human Shigella infections in Henan Province, China, in 2006
Abstract
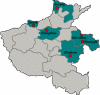
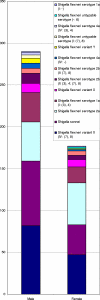
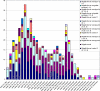
In 2006, 3,531 fecal samples were collected from patients with diarrhea in Henan Province, China. A total of 467 (13.2%) Shigella strains were isolated and serotyped. Seventy-one Shigella flexneri strains were characterized by MIC determination, pulsed-field gel electrophoresis (PFGE), and detection of genes encoding cephalosporin resistance. Most infections were caused by S. flexneri variant X [IV:(7),8] (27.6%), S. sonnei (24.2%), and S. flexneri 2a (20.8%). However, large regional differences were observed. Significantly higher odds (2.0) of females compared to males were infected with S. flexneri 2a. Untypeable S. flexneri (-:6) isolates were absent among males, as were untypeable S. flexneri [I:(7),8] isolates among females. Patient ages ranged from 2 months to 82 years, with 231 subjects (49.7%) <5 years of age. Most of the patients were male (62.1% [n = 290]). Infections peaked in July; week 27 with 38 cases (8.1%). All of the 71 S. flexneri conferred resistance to nalidixic acid; in addition, 21% (n = 15) and 79% (n = 56) were high- and low-level resistant to ciprofloxacin, respectively. Six S. flexneri isolates {serotype 2b [II:7,(8)] and 2b [II:(3),4;7,(8)]} harbored the bla(CTX-M-14) or bla(CTX-M-15) gene. A total of 52 unique XbaI PFGE patterns were observed among the 71 S. flexneri isolates with 11 distinct PFGE clusters. This study revealed a high prevalence of shigellosis with geographical differences in the distribution of serotypes in the distribution of serotypes and also differences in comparisons by gender. A high frequency of resistance, including 100% resistance to ciprofloxacin and resistance to extended-spectrum cephalosporins, was observed. We detected several isolates exhibiting the same PFGE type and MIC profile, indicating multiple undetected outbreaks.
Figures

References
-
- Andres, P., A. Petroni, D. Faccone, F. Pasteran, R. Melano, M. Rapoport, M. Martinez, C. Culasso, B. A. Di, B. Irigoyen, J. Mulki, A. Procopio, S. M. von, and M. Galas. 2005. Extended-spectrum beta-lactamases in Shigella flexneri from Argentina: first report of TOHO-1 outside Japan. Int. J. Antimicrob. Agents 25:501-507. - PubMed
-
- Bangtrakulnonth, A., A. R. Vieira, D. M. Lo Fo Wong, S. Pornreongwong, C. Pulsrikarn, P. Sawanpanyalert, R. S. Hendriksen, and F. M. Aarestrup. 2008. Shigella from humans in Thailand during 1993 to 2006: spatial-time trends in species and serotype distribution. Foodborne. Pathog. Dis. 5:773-784. - PubMed
-
- Bennish, M. L., and B. J. Wojtyniak. 1991. Mortality due to shigellosis: community and hospital data. Rev. Infect. Dis. 13(Suppl. 4):S245-S251. - PubMed
-
- Bopp, C. A., F. W. Brenner, P. I. Fields, J. G. Wells, and N. A. Strockbine. 2003. Escherichia, Shigella, and Salmonella, p. 654-671. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. ASM Press, Washington, DC.
-
- Collignon, P., J. H. Powers, T. M. Chiller, A. Kidara-Kane, and F. M. Aarestrup. 2009. World Health Organization ranking of antimicrobials according to their importance in human medicine: a critical step for developing risk management strategies for the use of antimicrobials in food production animals. Clin. Infect. Dis. 49:132-141. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

