Toward the second generation of optogenetic tools
- PMID: 21068304
- PMCID: PMC2997431
- DOI: 10.1523/JNEUROSCI.4190-10.2010
Toward the second generation of optogenetic tools
Abstract
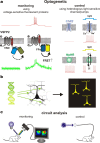
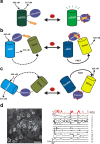
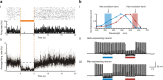
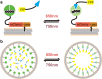
This mini-symposium aims to provide an integrated perspective on recent developments in optogenetics. Research in this emerging field combines optical methods with targeted expression of genetically encoded, protein-based probes to achieve experimental manipulation and measurement of neural systems with superior temporal and spatial resolution. The essential components of the optogenetic toolbox consist of two kinds of molecular devices: actuators and reporters, which respectively enable light-mediated control or monitoring of molecular processes. The first generation of genetically encoded calcium reporters, fluorescent proteins, and neural activators has already had a great impact on neuroscience. Now, a second generation of voltage reporters, neural silencers, and functionally extended fluorescent proteins hold great promise for continuing this revolution. In this review, we will evaluate and highlight the limitations of presently available optogenic tools and discuss where these technologies and their applications are headed in the future.
Figures
References
-
- Ai HW, Shaner NC, Cheng Z, Tsien RY, Campbell RE. Exploration of new chromophore structures leads to the identification of improved blue fluorescent proteins. Biochemistry. 2007;46:5904–5910. - PubMed
-
- Akemann W, Mutoh H, Perron A, Rossier J, Knöpfel T. Imaging brain electric signals with genetically targeted voltage-sensitive fluorescent proteins. Nat Methods. 2010;7:643–649. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
