IL-6- and NGF-induced rapid control of protein synthesis and nociceptive plasticity via convergent signaling to the eIF4F complex
- PMID: 21068317
- PMCID: PMC3056511
- DOI: 10.1523/JNEUROSCI.3947-10.2010
IL-6- and NGF-induced rapid control of protein synthesis and nociceptive plasticity via convergent signaling to the eIF4F complex
Abstract
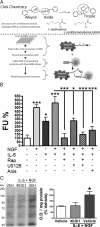
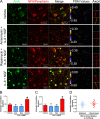
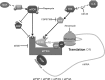
Despite the emergence of translational control pathways as mediators of nociceptive sensitization, effector molecules and mechanisms responsible for modulating activity in these pathways in pain conditions are largely unknown. We demonstrate that two major algogens, the cytokine interleukin 6 (IL-6) and the neurotrophin nerve growth factor (NGF), which are intimately linked to nociceptive plasticity across preclinical models and human pain conditions, signal primarily through two distinct pathways to enhance translation in sensory neurons by converging onto the eukaryotic initiation factor (eIF) eIF4F complex. We directly demonstrate that the net result of IL-6 and NGF signaling is an enhancement of eIF4F complex formation and an induction of nascent protein synthesis in primary afferent neurons and their axons. Moreover, IL-6- and NGF-induced mechanical nociceptive plasticity is blocked by inhibitors of general and cap-dependent protein synthesis. These results establish IL-6- and NGF-mediated cap-dependent translation of local proteins as a new model for nociceptive plasticity.
Figures




Similar articles
-
Resveratrol engages AMPK to attenuate ERK and mTOR signaling in sensory neurons and inhibits incision-induced acute and chronic pain.Mol Pain. 2012 Jan 23;8:5. doi: 10.1186/1744-8069-8-5. Mol Pain. 2012. PMID: 22269797 Free PMC article.
-
Bidirectional regulation of P body formation mediated by eIF4F complex formation in sensory neurons.Neurosci Lett. 2014 Mar 20;563:169-74. doi: 10.1016/j.neulet.2013.09.048. Epub 2013 Sep 27. Neurosci Lett. 2014. PMID: 24080374 Free PMC article.
-
Glucose and amino acids modulate translation factor activation by growth factors in PC12 cells.Biochem J. 2000 Apr 15;347(Pt 2):399-406. doi: 10.1042/0264-6021:3470399. Biochem J. 2000. PMID: 10749669 Free PMC article.
-
Molecular mechanisms of sensitization of pain-transducing P2X3 receptors by the migraine mediators CGRP and NGF.Mol Neurobiol. 2008 Feb;37(1):83-90. doi: 10.1007/s12035-008-8020-5. Epub 2008 May 6. Mol Neurobiol. 2008. PMID: 18459072 Review.
-
Unraveling the story of NGF-mediated sensitization of nociceptive sensory neurons: ON or OFF the Trks?Mol Interv. 2007 Feb;7(1):26-41. doi: 10.1124/mi.7.1.6. Mol Interv. 2007. PMID: 17339604 Review.
Cited by
-
mTORC1 inhibition induces pain via IRS-1-dependent feedback activation of ERK.Pain. 2013 Jul;154(7):1080-91. doi: 10.1016/j.pain.2013.03.021. Epub 2013 Mar 15. Pain. 2013. PMID: 23607966 Free PMC article.
-
The potent, indirect adenosine monophosphate- activated protein kinase activator R419 attenuates mitogen-activated protein kinase signaling, inhibits nociceptor excitability, and reduces pain hypersensitivity in mice.Pain Rep. 2016 Jul;1(1):e562. doi: 10.1097/PR9.0000000000000562. Pain Rep. 2016. PMID: 27672681 Free PMC article.
-
Roles of AMPK and Its Downstream Signals in Pain Regulation.Life (Basel). 2021 Aug 16;11(8):836. doi: 10.3390/life11080836. Life (Basel). 2021. PMID: 34440581 Free PMC article. Review.
-
Mast cell-derived chymases are essential for the resolution of inflammatory pain in mice.Pain. 2025 Mar 4;166(8):1811-1822. doi: 10.1097/j.pain.0000000000003565. Pain. 2025. PMID: 40035664
-
gp130 cytokines are positive signals triggering changes in gene expression and axon outgrowth in peripheral neurons following injury.Front Mol Neurosci. 2012 Jan 20;4:62. doi: 10.3389/fnmol.2011.00062. eCollection 2011. Front Mol Neurosci. 2012. PMID: 22319466 Free PMC article.
References
-
- Akira S, Yoshida K, Tanaka T, Taga T, Kishimoto T. Targeted disruption of the IL-6 related genes: gp130 and NF-IL-6. Immunol Rev. 1995;148:221–253. - PubMed
-
- Amaya F, Wang H, Costigan M, Allchorne AJ, Hatcher JP, Egerton J, Stean T, Morisset V, Grose D, Gunthorpe MJ, Chessell IP, Tate S, Green PJ, Woolf CJ. The voltage-gated sodium channel Na(v)1.9 is an effector of peripheral inflammatory pain hypersensitivity. J Neurosci. 2006;26:12852–12860. - PMC - PubMed
-
- Anderson LC, Rao RD. Interleukin-6 and nerve growth factor levels in peripheral nerve and brainstem after trigeminal nerve injury in the rat. Arch Oral Biol. 2001;46:633–640. - PubMed
-
- Andratsch M, Mair N, Constantin CE, Scherbakov N, Benetti C, Quarta S, Vogl C, Sailer CA, Uceyler N, Brockhaus J, Martini R, Sommer C, Zeilhofer HU, Müller W, Kuner R, Davis JB, Rose-John S, Kress M. A key role for gp130 expressed on peripheral sensory nerves in pathological pain. J Neurosci. 2009;29:13473–13483. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
