Dendritic sodium channels regulate network integration in globus pallidus neurons: a modeling study
- PMID: 21068320
- PMCID: PMC3022226
- DOI: 10.1523/JNEUROSCI.2662-10.2010
Dendritic sodium channels regulate network integration in globus pallidus neurons: a modeling study
Abstract
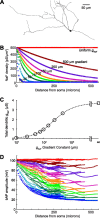
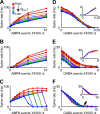
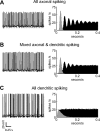
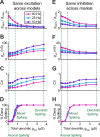
The globus pallidus (GP) predominantly contains GABAergic projection neurons that occupy a central position in the indirect pathway of the basal ganglia. They have long dendrites that can extend through one-half the diameter of the GP in rats, potentially enabling convergence and interaction between segregated basal ganglia circuits. Because of the length and fine diameter of GP dendrites, however, it is unclear how much influence distal synapses have on spiking activity. Dendritic expression of fast voltage-dependent Na(+) channels (NaF channels) can enhance the importance of distal excitatory synapses by allowing for dendritic spike initiation and by subthreshold boosting of EPSPs. Antibody labeling has demonstrated the presence of NaF channel proteins in GP dendrites, but the quantitative expression density of the channels remains unknown. We built a series of nine GP neuron models that differed only in their dendritic NaF channel expression level to assess the functional impact of this parameter. The models were all similar in their basic electrophysiological features; however, higher expression levels of dendritic NaF channels increased the relative effectiveness of distal inputs for both excitatory and inhibitory synapses, broadening the effective extent of the dendritic tree. Higher dendritic NaF channel expression also made the neurons more resistant to tonic inhibition and highly sensitive to clustered synchronous excitation. The dendritic NaF channel expression pattern may therefore be a critical determinant of convergence for both the striatopallidal and subthalamopallidal projections, while also dictating which spatiotemporal input patterns are most effective at driving GP neuron output.
Figures


Similar articles
-
Dendritic sodium channels promote active decorrelation and reduce phase locking to parkinsonian input oscillations in model globus pallidus neurons.J Neurosci. 2011 Jul 27;31(30):10919-36. doi: 10.1523/JNEUROSCI.6062-10.2011. J Neurosci. 2011. PMID: 21795543 Free PMC article.
-
Sodium channels and dendritic spike initiation at excitatory synapses in globus pallidus neurons.J Neurosci. 2004 Jan 14;24(2):329-40. doi: 10.1523/JNEUROSCI.3937-03.2004. J Neurosci. 2004. PMID: 14724231 Free PMC article.
-
Phase response curve analysis of a full morphological globus pallidus neuron model reveals distinct perisomatic and dendritic modes of synaptic integration.J Neurosci. 2010 Feb 17;30(7):2767-82. doi: 10.1523/JNEUROSCI.3959-09.2010. J Neurosci. 2010. PMID: 20164360 Free PMC article.
-
Functional connectivity and integrative properties of globus pallidus neurons.Neuroscience. 2011 Dec 15;198:44-53. doi: 10.1016/j.neuroscience.2011.07.050. Epub 2011 Jul 27. Neuroscience. 2011. PMID: 21835227 Free PMC article. Review.
-
Striatal information signaling and integration in globus pallidus: timing matters.Neurosignals. 2005;14(6):281-9. doi: 10.1159/000093043. Neurosignals. 2005. PMID: 16772731 Review.
Cited by
-
Motor cortex can directly drive the globus pallidus neurons in a projection neuron type-dependent manner in the rat.Elife. 2019 Nov 12;8:e49511. doi: 10.7554/eLife.49511. Elife. 2019. PMID: 31711567 Free PMC article.
-
Synchronized firing of fast-spiking interneurons is critical to maintain balanced firing between direct and indirect pathway neurons of the striatum.J Neurophysiol. 2014 Feb;111(4):836-48. doi: 10.1152/jn.00382.2013. Epub 2013 Dec 4. J Neurophysiol. 2014. PMID: 24304860 Free PMC article.
-
Dendritic sodium channels promote active decorrelation and reduce phase locking to parkinsonian input oscillations in model globus pallidus neurons.J Neurosci. 2011 Jul 27;31(30):10919-36. doi: 10.1523/JNEUROSCI.6062-10.2011. J Neurosci. 2011. PMID: 21795543 Free PMC article.
-
Robustness, variability, phase dependence, and longevity of individual synaptic input effects on spike timing during fluctuating synaptic backgrounds: a modeling study of globus pallidus neuron phase response properties.Neuroscience. 2012 Sep 6;219:92-110. doi: 10.1016/j.neuroscience.2012.05.059. Epub 2012 Jun 1. Neuroscience. 2012. PMID: 22659567 Free PMC article.
-
The microcircuits of striatum in silico.Proc Natl Acad Sci U S A. 2020 Apr 28;117(17):9554-9565. doi: 10.1073/pnas.2000671117. Epub 2020 Apr 22. Proc Natl Acad Sci U S A. 2020. PMID: 32321828 Free PMC article.
References
-
- Bernard V, Bolam JP. Subcellular and subsynaptic distribution of the NR1 subunit of the NMDA receptor in the neostriatum and globus pallidus of the rat: co-localization at synapses with the GluR2/3 subunit of the AMPA receptor. Eur J Neurosci. 1998;10:3721–3736. - PubMed
-
- Burkhardt JM, Constantinidis C, Anstrom KK, Roberts DC, Woodward DJ. Synchronous oscillations and phase reorganization in the basal ganglia during akinesia induced by high-dose haloperidol. Eur J Neurosci. 2007;26:1912–1924. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
