Repeated swim impairs serotonin clearance via a corticosterone-sensitive mechanism: organic cation transporter 3, the smoking gun
- PMID: 21068324
- PMCID: PMC3064500
- DOI: 10.1523/JNEUROSCI.2740-10.2010
Repeated swim impairs serotonin clearance via a corticosterone-sensitive mechanism: organic cation transporter 3, the smoking gun
Abstract
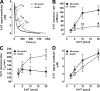
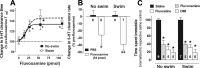
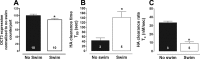
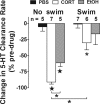
Activation of the hypothalamic-pituitary-adrenal (HPA) axis is associated with increased extracellular serotonin (5-HT) in limbic brain regions. The mechanism through which this occurs remains unclear. One way could be via HPA axis-dependent impairment of serotonin transporter (SERT) function, the high-affinity uptake mechanism for 5-HT. Consistent with this idea, we found that 5-HT clearance rate in hippocampus was dramatically reduced in mice exposed to repeated swim, a stimulus known to activate the HPA axis. However, this phenomenon also occurred in mice lacking SERT, ruling out SERT as a mechanism. The organic cation transporter 3 (OCT3) is emerging as an important regulator of brain 5-HT. Moreover, corticosterone, which is released upon HPA axis activation, blocks 5-HT uptake by OCT3. Repeated swim produced a persistent elevation in plasma corticosterone, and, consistent with prolonged blockade by corticosterone, we found that OCT3 expression and function were reduced in these mice. Importantly, this effect of repeated swim to reduce 5-HT clearance rate was corticosterone dependent, as evidenced by its absence in adrenalectomized mice, in which plasma corticosterone levels were essentially undetectable. Behaviorally, mice subjected to repeated swim spent less time immobile in the tail suspension test than control mice, but responded similarly to SERT- and norepinephrine transporter-selective antidepressants. Together, these results show that reduced 5-HT clearance following HPA axis activation is likely mediated, at least in part, by the corticosterone-sensitive OCT3, and that drugs developed to selectively target OCT3 (unlike corticosterone) may be candidates for the development of novel antidepressant medications.
Figures


Similar articles
-
Chronic administration of glucocorticoid receptor ligands increases anxiety-like behavior and selectively increase serotonin transporters in the ventral hippocampus.Brain Res. 2023 Feb 1;1800:148189. doi: 10.1016/j.brainres.2022.148189. Epub 2022 Nov 30. Brain Res. 2023. PMID: 36462646 Free PMC article.
-
Differential effects of acute and chronic social defeat stress on hypothalamic-pituitary-adrenal axis function and hippocampal serotonin release in mice.J Neuroendocrinol. 2006 May;18(5):330-8. doi: 10.1111/j.1365-2826.2006.01422.x. J Neuroendocrinol. 2006. PMID: 16629831
-
Altered serotonergic neurotransmission but normal hypothalamic-pituitary-adrenocortical axis activity in mice chronically treated with the corticotropin-releasing hormone receptor type 1 antagonist NBI 30775.Neuropsychopharmacology. 2003 Dec;28(12):2148-59. doi: 10.1038/sj.npp.1300267. Neuropsychopharmacology. 2003. PMID: 12915860
-
Behavioral and serotonergic consequences of decreasing or increasing hippocampus brain-derived neurotrophic factor protein levels in mice.Neuropharmacology. 2008 Nov;55(6):1006-14. doi: 10.1016/j.neuropharm.2008.08.001. Epub 2008 Aug 12. Neuropharmacology. 2008. PMID: 18761360 Review.
-
Roles for the uptake2 transporter OCT3 in regulation of dopaminergic neurotransmission and behavior.Neurochem Int. 2019 Feb;123:46-49. doi: 10.1016/j.neuint.2018.07.008. Epub 2018 Jul 25. Neurochem Int. 2019. PMID: 30055194 Free PMC article. Review.
Cited by
-
Stress produces aversion and potentiates cocaine reward by releasing endogenous dynorphins in the ventral striatum to locally stimulate serotonin reuptake.J Neurosci. 2012 Dec 5;32(49):17582-96. doi: 10.1523/JNEUROSCI.3220-12.2012. J Neurosci. 2012. PMID: 23223282 Free PMC article.
-
Organic cation transporter 3: A cellular mechanism underlying rapid, non-genomic glucocorticoid regulation of monoaminergic neurotransmission, physiology, and behavior.Horm Behav. 2018 Aug;104:173-182. doi: 10.1016/j.yhbeh.2018.05.003. Epub 2018 May 10. Horm Behav. 2018. PMID: 29738736 Free PMC article. Review.
-
Ethanol inhibits dopamine uptake via organic cation transporter 3: Implications for ethanol and cocaine co-abuse.Mol Psychiatry. 2023 Jul;28(7):2934-2945. doi: 10.1038/s41380-023-02064-5. Epub 2023 Jun 13. Mol Psychiatry. 2023. PMID: 37308680 Free PMC article.
-
Aging and stress: past hypotheses, present approaches and perspectives.Aging Dis. 2011 Feb;2(1):80-99. Epub 2011 Jan 28. Aging Dis. 2011. PMID: 22396868 Free PMC article.
-
Chronic administration of glucocorticoid receptor ligands increases anxiety-like behavior and selectively increase serotonin transporters in the ventral hippocampus.Brain Res. 2023 Feb 1;1800:148189. doi: 10.1016/j.brainres.2022.148189. Epub 2022 Nov 30. Brain Res. 2023. PMID: 36462646 Free PMC article.
References
-
- Alnouti Y, Petrick JS, Klaassen CD. Tissue distribution and ontogeny of organic cation transporters in mice. Drug Metab Dispos. 2006;34:477–482. - PubMed
-
- Amat J, Matus-Amat P, Watkins LR, Maier SF. Escapable and inescapable stress differentially and selectively alter extracellular levels of 5-HT in the ventral hippocampus and dorsal periaqueductal gray of the rat. Brain Res. 1998;797:12–22. - PubMed
-
- Baganz NL, Horton RE, Calderon AS, Owens WA, Munn JL, Watts LT, Koldzic-Zivanovic N, Jeske NA, Koek W, Toney GM, Daws LC. Organic cation transporter 3: keeping the brake on extracellular serotonin in serotonin transporter deficient mice. Proc Natl Acad Sci U S A. 2008;105:18976–18981. - PMC - PubMed
-
- Beekman M, Flachskamm C, Linthorst AC. Effects of exposure to a predator on behaviour and serotonergic neurotransmission in different brain regions of C57bl/6N mice. Eur J Neurosci. 2005;21:2825–2836. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
