Activation of microglia depends on Na+/H+ exchange-mediated H+ homeostasis
- PMID: 21068326
- PMCID: PMC3010222
- DOI: 10.1523/JNEUROSCI.3950-10.2010
Activation of microglia depends on Na+/H+ exchange-mediated H+ homeostasis
Abstract
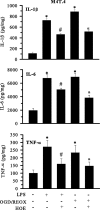
H(+) extrusion is important for sustained NADPH oxidase activation after "respiratory" burst in macrophage/microglia activation. In this study, we investigated the role of Na(+)/H(+) exchanger isoform 1 (NHE-1) in activation of microglia after lipopolysaccharide (LPS) or oxygen and glucose deprivation and reoxygenation (OGD/REOX) exposure. NHE-1 functioned in maintaining basal pH(i) of immortalized M4T.4 microglia or mouse primary microglia. Pharmacological inhibition of NHE-1 activity with the potent inhibitor cariporide [HOE 642 (4-isopropyl-3-methylsulfonyl-benzoyl-guanidine-methanesulfonate)] abolished pH(i) regulation in microglia under basal conditions. Activation of microglia either by LPS, phorbol myristate acetate, or OGD/REOX accelerated pH(i) regulation and caused pH(i) elevation, which was accompanied with an increase in [Na(+)](i) and [Ca(2+)](i) as well as production of superoxide anion and cytokines. Interestingly, inhibition of NHE-1 not only abolished pH(i) regulation but also reduced production of superoxide anion as well as expression of cytokines and inducible nitric oxide synthase. Together, these results reveal that there was a concurrent activation of NHE-1 in microglia in response to proinflammatory stimuli. The study suggests that NHE-1 functions to maintain microglial pH(i) homeostasis allowing for sustained NADPH oxidase function and "respiratory" burst.
Figures

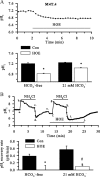
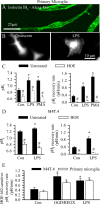

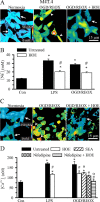

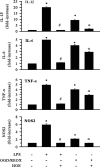


References
-
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. - PubMed
-
- Block ML, Hong JS. Chronic microglial activation and progressive dopaminergic neurotoxicity. Biochem Soc Trans. 2007;35:1127–1132. - PubMed
-
- Boscia F, Gala R, Pannaccione A, Secondo A, Scorziello A, Di Renzo G, Annunziato L. NCX1 expression and functional activity increase in microglia invading the infarct core. Stroke. 2009;40:3608–3617. - PubMed
-
- Boyarsky G, Ransom B, Schlue WR, Davis MB, Boron WF. Intracellular pH regulation in single cultured astrocytes from rat forebrain. Glia. 1993;8:241–248. - PubMed
-
- Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–C833. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 HD003352/HD/NICHD NIH HHS/United States
- NS43802/NS/NINDS NIH HHS/United States
- UL1 RR025011/RR/NCRR NIH HHS/United States
- R01 NS056118/NS/NINDS NIH HHS/United States
- P30 HD03352/HD/NICHD NIH HHS/United States
- R01 NS048216/NS/NINDS NIH HHS/United States
- NS36736/NS/NINDS NIH HHS/United States
- NS56118/NS/NINDS NIH HHS/United States
- NS45048/NS/NINDS NIH HHS/United States
- R01 NS038118/NS/NINDS NIH HHS/United States
- R01NS048216/NS/NINDS NIH HHS/United States
- R01 NS043802/NS/NINDS NIH HHS/United States
- 1UL1RR025011/RR/NCRR NIH HHS/United States
- R01 NS036736/NS/NINDS NIH HHS/United States
- R01 NS045048/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous
