A neuronal population measure of attention predicts behavioral performance on individual trials
- PMID: 21068329
- PMCID: PMC3045704
- DOI: 10.1523/JNEUROSCI.2171-10.2010
A neuronal population measure of attention predicts behavioral performance on individual trials
Abstract
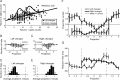
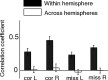
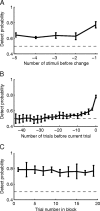
Visual attention improves perception for an attended location or feature and also modulates the responses of sensory neurons. In laboratory studies, the sensory stimuli and task instructions are held constant within an attentional condition, but despite experimenters' best efforts, attention likely varies from moment to moment. Because most previous studies have focused on single neurons, it has been impossible to use neuronal responses to identify attentional fluctuations and determine whether these are associated with changes in behavior. We show that an instantaneous measure of attention based on the responses of a modest number of neurons in area V4 of the rhesus monkey (Macaca mulatta) can reliably predict large changes in an animal's ability to perform a difficult psychophysical task. Unexpectedly, this measure shows that the amount of attention allocated at any moment to locations in opposite hemifields is uncorrelated, suggesting that animals allocate attention to each stimulus independently rather than moving their attentional focus from one location to another.
Figures




References
-
- Alvarez GA, Cavanagh P. Independent resources for attentional tracking in the left and right visual hemifields. Psychol Sci. 2005;16:637–643. - PubMed
-
- Assad JA. Neural coding of behavioral relevance in parietal cortex. Curr Opin Neurobiol. 2003;13:194–197. - PubMed
-
- Barberini CL, Cohen MR, Wandell BA, Newsome WT. Cone signal interactions in direction-selective neurons in the middle temporal visual area (MT) J Vis. 2005;5:603–621. - PubMed
-
- Boudreau CE, Williford TH, Maunsell JHR. Effects of task difficulty and target likelihood in area V4 of macaque monkeys. J Neurophysiol. 2006;96:2377–2387. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
