Gap junction expression is required for normal chemical synapse formation
- PMID: 21068332
- PMCID: PMC3478946
- DOI: 10.1523/JNEUROSCI.2331-10.2010
Gap junction expression is required for normal chemical synapse formation
Abstract
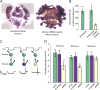
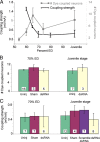
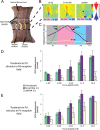
Electrical and chemical synapses provide two distinct modes of direct communication between neurons, and the embryonic development of the two is typically not simultaneous. Instead, in both vertebrates and invertebrates, gap junction-based electrical synapses arise before chemical synaptogenesis, and the early circuits composed of gap junction-based electrical synapses resemble those produced later by chemical synapses. This developmental sequence from electrical to chemical synapses has led to the hypothesis that, in developing neuronal circuits, electrical junctions are necessary forerunners of chemical synapses. Up to now, it has been difficult to test this hypothesis directly, but we can identify individual neurons in the leech nervous system from before the time when synapses are first forming, so we could test the hypothesis. Using RNA interference, we transiently reduced gap junction expression in individual identified neurons during the 2-4 d when chemical synapses normally form. We found that the expected chemical synapses failed to form on schedule, and they were still missing months later when the nervous system was fully mature. We conclude that the formation of gap junctions between leech neurons is a necessary step in the formation of chemical synaptic junctions, confirming the predicted relation between electrical synapses and chemical synaptogenesis.
Figures




Similar articles
-
The components of an electrical synapse as revealed by expansion microscopy of a single synaptic contact.Elife. 2024 Jul 12;13:e91931. doi: 10.7554/eLife.91931. Elife. 2024. PMID: 38994821 Free PMC article.
-
CNQX and AMPA inhibit electrical synaptic transmission: a potential interaction between electrical and glutamatergic synapses.Brain Res. 2008 Sep 4;1228:43-57. doi: 10.1016/j.brainres.2008.06.035. Epub 2008 Jun 20. Brain Res. 2008. PMID: 18601913 Free PMC article.
-
Photoreceptive Ganglion Cells Drive Circuits for Local Inhibition in the Mouse Retina.J Neurosci. 2021 Feb 17;41(7):1489-1504. doi: 10.1523/JNEUROSCI.0674-20.2020. Epub 2021 Jan 4. J Neurosci. 2021. PMID: 33397711 Free PMC article.
-
Electrical synapses--gap junctions in the brain.Results Probl Cell Differ. 2006;43:99-128. doi: 10.1007/400_013. Results Probl Cell Differ. 2006. PMID: 17068969 Review.
-
Gap junctions and their implications for neurogenesis and maturation of synaptic circuitry in the developing neocortex.Results Probl Cell Differ. 2002;39:53-73. doi: 10.1007/978-3-540-46006-0_3. Results Probl Cell Differ. 2002. PMID: 12353468 Review.
Cited by
-
Distribution of the gap junction protein connexin 35 in the central nervous system of developing zebrafish larvae.Front Neural Circuits. 2013 May 14;7:91. doi: 10.3389/fncir.2013.00091. eCollection 2013. Front Neural Circuits. 2013. PMID: 23717264 Free PMC article.
-
Regulatory Roles of Metabotropic Glutamate Receptors on Synaptic Communication Mediated by Gap Junctions.Neuroscience. 2021 Feb 21;456:85-94. doi: 10.1016/j.neuroscience.2020.06.034. Epub 2020 Jun 30. Neuroscience. 2021. PMID: 32619474 Free PMC article. Review.
-
Shaking-B misexpression increases the formation of gap junctions but not chemical synapses between auditory sensory neurons and the giant fiber of Drosophila melanogaster.PLoS One. 2018 Aug 17;13(8):e0198710. doi: 10.1371/journal.pone.0198710. eCollection 2018. PLoS One. 2018. PMID: 30118493 Free PMC article.
-
Gjd2b-mediated gap junctions promote glutamatergic synapse formation and dendritic elaboration in Purkinje neurons.Elife. 2021 Aug 4;10:e68124. doi: 10.7554/eLife.68124. Elife. 2021. PMID: 34346310 Free PMC article.
-
The role of retinoic acid in the formation and modulation of invertebrate central synapses.J Neurophysiol. 2017 Feb 1;117(2):692-704. doi: 10.1152/jn.00737.2016. Epub 2016 Nov 16. J Neurophysiol. 2017. PMID: 27852736 Free PMC article.
References
-
- Baca SM, Thomson EE, Kristan WB., Jr Location and intensity discrimination in the leech local bend response quantified using optic flow and principal components analysis. J Neurophysiol. 2005;93:3560–3572. - PubMed
-
- Bani-Yaghoub M, Underhill TM, Naus CC. Gap junction blockage interferes with neuronal and astroglial differentiation of mouse P19 embryonal carcinoma cells. Dev Genet. 1999a;24:69–81. - PubMed
-
- Bani-Yaghoub M, Bechberger JF, Underhill TM, Naus CC. The effects of gap junction blockage on neuronal differentiation of human NTera2/clone D1 cells. Exp Neurol. 1999b;156:16–32. - PubMed
-
- Bennett MV. Physiology of electrotonic junctions. Ann NY Acad Sci. 1966;137:509–539. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
