Flotillin-mediated endocytic events dictate cell type-specific responses to semaphorin 3A
- PMID: 21068336
- PMCID: PMC3496384
- DOI: 10.1523/JNEUROSCI.1821-10.2010
Flotillin-mediated endocytic events dictate cell type-specific responses to semaphorin 3A
Abstract
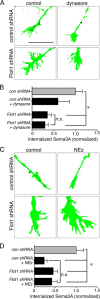
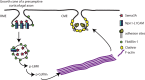
Cortical efferents growing in the same environment diverge early in development. The expression of particular transcription factors dictates the trajectories taken, presumably by regulating responsiveness to guidance cues via cellular mechanisms that are not yet known. Here, we show that cortical neurons that are dissociated and grown in culture maintain their cell type-specific identities defined by the expression of transcription factors. Using this model system, we sought to identify and characterize mechanisms that are recruited to produce cell type-specific responses to Semaphorin 3A (Sema3A), a guidance cue that would be presented similarly to cortical axons in vivo. Axons from presumptive corticofugal neurons lacking the transcription factor Satb2 and expressing Ctip2 or Tbr1 respond far more robustly to Sema3A than those from presumptive callosal neurons expressing Satb2. Both populations of axons express similar levels of Sema3A receptors (neuropilin-1, cell adhesion molecule L1, and plexinA4), but significantly, axons from neurons lacking Satb2 internalize more Sema3A, and they do so via a raft-mediated endocytic pathway. We used an in silico approach to identify the endocytosis effector flotillin-1 as a Sema3A signaling candidate. We tested the contributions of flotillin-1 to Sema3A endocytosis and signaling, and show that raft-mediated Sema3A endocytosis is defined by and depends on the recruitment of flotillin-1, which mediates LIM domain kinase activation and regulates axon responsiveness to Sema3A in presumptive corticofugal axons.
Figures






Similar articles
-
FAK-MAPK-dependent adhesion disassembly downstream of L1 contributes to semaphorin3A-induced collapse.EMBO J. 2008 Jun 4;27(11):1549-62. doi: 10.1038/emboj.2008.86. Epub 2008 May 8. EMBO J. 2008. PMID: 18464795 Free PMC article.
-
Semaphorin 3A is a retrograde cell death signal in developing sympathetic neurons.Development. 2016 May 1;143(9):1560-70. doi: 10.1242/dev.134627. Development. 2016. PMID: 27143756 Free PMC article.
-
Semaphorin 3A activates the guanosine triphosphatase Rab5 to promote growth cone collapse and organize callosal axon projections.Sci Signal. 2014 Aug 26;7(340):ra81. doi: 10.1126/scisignal.2005334. Sci Signal. 2014. PMID: 25161316 Free PMC article.
-
Molecular basis of semaphorin-mediated axon guidance.J Neurobiol. 2000 Aug;44(2):219-29. doi: 10.1002/1097-4695(200008)44:2<219::aid-neu11>3.0.co;2-w. J Neurobiol. 2000. PMID: 10934324 Review.
-
The CRMP family of proteins and their role in Sema3A signaling.Adv Exp Med Biol. 2007;600:1-11. doi: 10.1007/978-0-387-70956-7_1. Adv Exp Med Biol. 2007. PMID: 17607942 Free PMC article. Review.
Cited by
-
Flotillins regulate membrane mobility of the dopamine transporter but are not required for its protein kinase C dependent endocytosis.Traffic. 2013 Jun;14(6):709-24. doi: 10.1111/tra.12059. Epub 2013 Mar 11. Traffic. 2013. PMID: 23418867 Free PMC article.
-
The Chemorepulsive Protein Semaphorin 3A and Perineuronal Net-Mediated Plasticity.Neural Plast. 2016;2016:3679545. doi: 10.1155/2016/3679545. Epub 2016 Jan 14. Neural Plast. 2016. PMID: 27057361 Free PMC article. Review.
-
Endocytosis and endosomes at the crossroads of regulating trafficking of axon outgrowth-modifying receptors.Traffic. 2011 Sep;12(9):1099-108. doi: 10.1111/j.1600-0854.2011.01213.x. Epub 2011 May 23. Traffic. 2011. PMID: 21535338 Free PMC article. Review.
-
Designer nanoparticle: nanobiotechnology tool for cell biology.Nano Converg. 2016;3(1):22. doi: 10.1186/s40580-016-0082-x. Epub 2016 Sep 15. Nano Converg. 2016. PMID: 28191432 Free PMC article. Review.
-
Membrane trafficking in podocyte health and disease.Pediatr Nephrol. 2013 Sep;28(9):1723-37. doi: 10.1007/s00467-012-2281-y. Epub 2012 Aug 30. Pediatr Nephrol. 2013. PMID: 22932996 Free PMC article. Review.
References
-
- Aizawa H, Wakatsuki S, Ishii A, Moriyama K, Sasaki Y, Ohashi K, Sekine-Aizawa Y, Sehara-Fujisawa A, Mizuno K, Goshima Y, Yahara I. Phosphorylation of cofilin by LIM-kinase is necessary for semaphorin 3A-induced growth cone collapse. Nat Neurosci. 2001;4:367–373. - PubMed
-
- Alcamo EA, Chirivella L, Dautzenberg M, Dobreva G, Fariñas I, Grosschedl R, McConnell SK. Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron. 2008;57:364–377. - PubMed
-
- Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, Macklis JD. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45:207–221. - PubMed
-
- Bagnard D, Lohrum M, Uziel D, Püschel AW, Bolz J. Semaphorins act as attractive and repulsive guidance signals during the development of cortical projections. Development. 1998;125:5043–5053. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
