The phosphorylation state of MRLC is polyamine dependent in intestinal epithelial cells
- PMID: 21068360
- PMCID: PMC3023191
- DOI: 10.1152/ajpcell.00247.2010
The phosphorylation state of MRLC is polyamine dependent in intestinal epithelial cells
Abstract
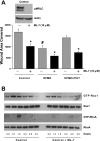
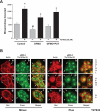
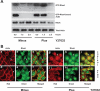
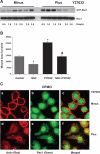
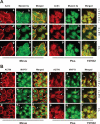
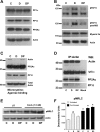
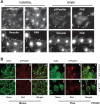
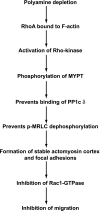
Cell migration is important to the integrity of the gastrointestinal tract for the normal movement of cells from crypt to villi and the healing of wounds. Polyamines are essential to cell migration, mucosal restitution, and, hence, healing. Polyamine depletion by α-difluoromethyl ornithine (DFMO) inhibited migration by decreasing lamellipodia and stress fiber formation and preventing the activation of Rho-GTPases. Polyamine depletion increased the association of the thick F-actin cortex with phosphorylated myosin regulatory light chain (pMRLC). In this study, we determined why MRLC is constitutively phosphorylated as part of the actin cortex. Inhibition of myosin light chain kinase (MLCK) decreased RhoA and Rac1 activities and significantly inhibited migration. Polyamine depletion increased phosphorylation of MRLC (Thr18/Ser19) and stabilized the actin cortex and focal adhesions. The Rho-kinase inhibitor Y27632 increased spreading and migration by decreasing the phosphorylation of MRLC, remodeling focal adhesions, and by activating Rho-GTPases. Thus phosphorylation of MRLC appears to be the rate-limiting step during the migration of IEC-6 cells. In addition, increased localization of RhoA with the actin cortex in polyamine-depleted cells appears to activate Rho-kinase. In the absence of polyamines, activated Rho-kinase phosphorylates myosin phosphatase targeting subunit 1 (MYPT1) at serine-668 leading to its inactivation and preventing the recruitment of phosphatase (protein phosphastase, PP1cδ) to the actomyosin cortex. In this condition, MRLC is constitutively phosphorylated and cycling does not occur. Thus activated myosin binds F-actin stress fibers and prevents focal adhesion turnover, Rho-GTPase activation, and the remodeling of the cytoskeleton required for migration.
Figures

References
-
- Alessi D, MacDougall LK, Sola MM, Ikebe M, Cohen P. The control of protein phosphatase-1 by targeting subunits. The major myosin phosphatase in avian smooth muscle is a novel form of protein phosphatase-1. Eur J Biochem 1210: 1023–1035, 1992 - PubMed
-
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem 271: 20246–20249, 1996 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

