Tuning the reactivity of semiconductor surfaces by functionalization with amines of different basicity
- PMID: 21068370
- PMCID: PMC3024700
- DOI: 10.1073/pnas.1006656107
Tuning the reactivity of semiconductor surfaces by functionalization with amines of different basicity
Abstract
Surface functionalization of semiconductors has been the backbone of the newest developments in microelectronics, energy conversion, sensing device design, and many other fields of science and technology. Over a decade ago, the notion of viewing the surface itself as a chemical reagent in surface reactions was introduced, and adding a variety of new functionalities to the semiconductor surface has become a target of research for many groups. The electronic effects on the substrate have been considered as an important consequence of chemical modification. In this work, we shift the focus to the electronic properties of the functional groups attached to the surface and their role on subsequent reactivity. We investigate surface functionalization of clean Si(100)-2 × 1 and Ge(100)-2 × 1 surfaces with amines as a way to modify their reactivity and to fine tune this reactivity by considering the basicity of the attached functionality. The reactivity of silicon and germanium surfaces modified with ethylamine (CH(3)CH(2)NH(2)) and aniline (C(6)H(5)NH(2)) is predicted using density functional theory calculations of proton attachment to the nitrogen of the adsorbed amine to differ with respect to a nucleophilic attack of the surface species. These predictions are then tested using a model metalorganic reagent, tetrakis(dimethylamido)titanium (((CH(3))(2)N)(4)Ti, TDMAT), which undergoes a transamination reaction with sufficiently nucleophilic amines, and the reactivity tests confirm trends consistent with predicted basicities. The identity of the underlying semiconductor surface has a profound effect on the outcome of this reaction, and results comparing silicon and germanium are discussed.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
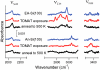

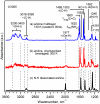



Similar articles
-
Functionalization of the semiconductor surfaces of diamond (100), Si (100), and Ge (100) by cycloaddition of transition metal oxides: a theoretical prediction.Langmuir. 2009 Sep 1;25(17):9840-6. doi: 10.1021/la900942e. Langmuir. 2009. PMID: 19499936
-
Artificial atoms on semiconductor surfaces.Proc Natl Acad Sci U S A. 2011 Jan 18;108(3):965-70. doi: 10.1073/pnas.1006665107. Epub 2010 Nov 19. Proc Natl Acad Sci U S A. 2011. PMID: 21097704 Free PMC article.
-
Block copolymer-templated chemistry on Si, Ge, InP, and GaAs surfaces.J Am Chem Soc. 2005 Jun 29;127(25):8932-3. doi: 10.1021/ja052281m. J Am Chem Soc. 2005. PMID: 15969553
-
Biochemistry and semiconductor electronics--the next big hit for silicon?J Phys Condens Matter. 2012 Apr 25;24(16):164201. doi: 10.1088/0953-8984/24/16/164201. Epub 2012 Mar 30. J Phys Condens Matter. 2012. PMID: 22465874 Free PMC article. Review.
-
Unlocking Germanium Potential: Stabilization Strategies Through Wet Chemical Functionalization.Materials (Basel). 2024 Dec 23;17(24):6285. doi: 10.3390/ma17246285. Materials (Basel). 2024. PMID: 39769886 Free PMC article. Review.
Cited by
-
Surface chemistry: key to control and advance myriad technologies.Proc Natl Acad Sci U S A. 2011 Jan 18;108(3):911-6. doi: 10.1073/pnas.1006671107. Proc Natl Acad Sci U S A. 2011. PMID: 21245359 Free PMC article.
-
Monolayer of Hydrazine Facilitates the Direct Covalent Attachment of C60 Fullerene to a Silicon Surface.Langmuir. 2017 Sep 5;33(35):8632-8639. doi: 10.1021/acs.langmuir.6b03975. Epub 2017 Feb 13. Langmuir. 2017. PMID: 28157324 Free PMC article.
-
Dynamics of Functionalized Surface Molecular Monolayers Studied with Ultrafast Infrared Vibrational Spectroscopy.J Phys Chem C Nanomater Interfaces. 2012 Nov 8;116(44):23428-23440. doi: 10.1021/jp307677b. J Phys Chem C Nanomater Interfaces. 2012. PMID: 23259027 Free PMC article.
References
-
- Yaffe O, et al. Molecular electronics at metal/semiconductor junctions. Si inversion by sub-nanometer molecular films. Nano Lett. 2009;9:2390–2394. - PubMed
-
- Hanrath T, Korgel BA. Chemical surface passivation of Ge nanowires. J Am Chem Soc. 2004;126:15466–15472. - PubMed
-
- Bashouti MY, Tung RT, Haick H. Tuning the electrical properties of Si nanowire field-effect transistors by molecular engineering. Small. 2009;5:2761–2769. - PubMed
-
- Vasudevan S, et al. Controlling transistor threshold voltages using molecular dipoles. J Appl Phys. 2009;105:093703.
-
- Avouris P. Manipulation of matter at the atomic and molecular levels. Acc Chem Res. 1995;28:95–102.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

