Measurement of adherent cell mass and growth
- PMID: 21068372
- PMCID: PMC2996435
- DOI: 10.1073/pnas.1011365107
Measurement of adherent cell mass and growth
Abstract
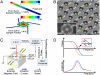
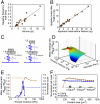
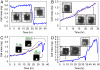
The characterization of physical properties of cells such as their mass and stiffness has been of great interest and can have profound implications in cell biology, tissue engineering, cancer, and disease research. For example, the direct dependence of cell growth rate on cell mass for individual adherent human cells can elucidate the mechanisms underlying cell cycle progression. Here we develop an array of micro-electro-mechanical systems (MEMS) resonant mass sensors that can be used to directly measure the biophysical properties, mass, and growth rate of single adherent cells. Unlike conventional cantilever mass sensors, our sensors retain a uniform mass sensitivity over the cell attachment surface. By measuring the frequency shift of the mass sensors with growing (soft) cells and fixed (stiff) cells, and through analytical modeling, we derive the Young's modulus of the unfixed cell and unravel the dependence of the cell mass measurement on cell stiffness. Finally, we grew individual cells on the mass sensors and measured their mass for 50+ hours. Our results demonstrate that adherent human colon epithelial cells have increased growth rates with a larger cell mass, and the average growth rate increases linearly with the cell mass, at 3.25%/hr. Our sensitive mass sensors with a position-independent mass sensitivity can be coupled with microscopy for simultaneous monitoring of cell growth and status, and provide an ideal method to study cell growth, cell cycle progression, differentiation, and apoptosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Killander D, Zetterberg A. Quantitative cytochemical studies on interphase growth. I. Determination of DNA, RNA and mass content of age determined mouse fibroblasts in vitro and of intercellular variation in generation time. Exp Cell Res. 1965;38:272–284. - PubMed
-
- Mitchison JM. Growth during the cell cycle. Int Rev Cytol. 2003;226:165–258. - PubMed
-
- Zetterberg A, Killander D. Quantitative cytochemical studies on interphase growth. 2. Derivation of synthesis curves from distribution of DNA, RNA and mass values of individual mouse fibroblasts in vitro. Exp Cell Res. 1965;39:22–32. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

