Anti-inflammatory effect of MAPK phosphatase-1 local gene transfer in inflammatory bone loss
- PMID: 21068780
- PMCID: PMC3086452
- DOI: 10.1038/gt.2010.139
Anti-inflammatory effect of MAPK phosphatase-1 local gene transfer in inflammatory bone loss
Abstract
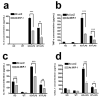
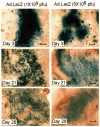
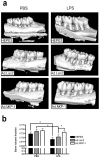
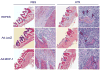
Alveolar bone loss associated with periodontal diseases is the result of osteoclastogenesis induced by bacterial pathogens. The mitogen-activated protein kinase (MAPK) phosphatase 1 (MKP-1) is a critical negative regulator of immune response as a key phosphatase capable of dephosphorylating activated MAPKs. In this study, rat macrophages transduced with recombinant adenovirus (Ad.)MKP-1 specifically dephosphorylated activated MAPKs induced by lipopolysaccharide (LPS) compared with control cells. Bone marrow macrophages from MKP-1 knockout (KO) mice exhibited higher interleukin (IL)-6, IL-10, tumor necrosis factor (TNF)-α, and select chemokine compared with wild-type (WT) mice when stimulated by LPS. In addition, bone marrow cultures from MKP-1 KO mice exhibited significantly more osteoclastogenesis induced by LPS than when compared with WT mice. Importantly, MKP-1 gene transfer in bone marrow cells of MKP-1 KO mice significantly decreased IL-6, IL-10, TNF-α and chemokine levels, and formed fewer osteoclasts induced by LPS than compared with control group of cells. Furthermore, MKP-1 gene transfer in an experimental periodontal disease model attenuated bone resorption induced by LPS. Histological analysis confirmed that periodontal tissues transduced with Ad. MKP-1 exhibited less infiltrated inflammatory cells, less osteoclasts and less IL-6 than compared with rats of control groups. These studies indicate that MKP-1 is a key therapeutic target to control of inflammation-induced bone loss.
Conflict of interest statement
The authors declare no conflict interest.
Figures



Similar articles
-
Critical role of MKP-1 in lipopolysaccharide-induced osteoclast formation through CXCL1 and CXCL2.Cytokine. 2015 Jan;71(1):71-80. doi: 10.1016/j.cyto.2014.08.007. Epub 2014 Sep 27. Cytokine. 2015. PMID: 25261746 Free PMC article.
-
MAP kinase phosphatase-1 protects against inflammatory bone loss.J Dent Res. 2009 Dec;88(12):1125-30. doi: 10.1177/0022034509349306. Epub 2009 Oct 28. J Dent Res. 2009. PMID: 19864641 Free PMC article.
-
MKP-1 regulates cytokine mRNA stability through selectively modulation subcellular translocation of AUF1.Cytokine. 2011 Nov;56(2):245-55. doi: 10.1016/j.cyto.2011.06.006. Epub 2011 Jul 5. Cytokine. 2011. PMID: 21733716 Free PMC article.
-
Mitogen-activated protein kinase phosphatase 2 regulates the inflammatory response in sepsis.Infect Immun. 2010 Jun;78(6):2868-76. doi: 10.1128/IAI.00018-10. Epub 2010 Mar 29. Infect Immun. 2010. PMID: 20351138 Free PMC article.
-
Diversity and specificity of the mitogen-activated protein kinase phosphatase-1 functions.Cell Mol Life Sci. 2013 Jan;70(2):223-37. doi: 10.1007/s00018-012-1041-2. Epub 2012 Jun 14. Cell Mol Life Sci. 2013. PMID: 22695679 Free PMC article. Review.
Cited by
-
Inhibition of acid sphingomyelinase by imipramine abolishes the synergy between metabolic syndrome and periodontitis on alveolar bone loss.J Periodontal Res. 2022 Jan;57(1):173-185. doi: 10.1111/jre.12951. Epub 2021 Nov 8. J Periodontal Res. 2022. PMID: 34748647 Free PMC article.
-
Periodontitis and diabetes interrelationships in rats: biochemical and histopathological variables.J Diabetes Metab Disord. 2019 May 16;18(1):163-172. doi: 10.1007/s40200-019-00403-4. eCollection 2019 Jun. J Diabetes Metab Disord. 2019. PMID: 31275887 Free PMC article.
-
Simvastatin inhibits lipopolysaccharide-induced osteoclastogenesis and reduces alveolar bone loss in experimental periodontal disease.J Periodontal Res. 2014 Aug;49(4):518-26. doi: 10.1111/jre.12132. Epub 2013 Oct 7. J Periodontal Res. 2014. PMID: 24117880 Free PMC article.
-
MAPK usage in periodontal disease progression.J Signal Transduct. 2012;2012:308943. doi: 10.1155/2012/308943. Epub 2012 Jan 23. J Signal Transduct. 2012. PMID: 22315682 Free PMC article.
-
Dual action of highbush blueberry proanthocyanidins on Aggregatibacter actinomycetemcomitans and the host inflammatory response.BMC Complement Altern Med. 2018 Jan 10;18(1):10. doi: 10.1186/s12906-017-2072-x. BMC Complement Altern Med. 2018. PMID: 29321009 Free PMC article.
References
-
- Brown LJ, Loe H. Prevalence, extent, severity and progression of periodontal disease. Periodontol 2000. 1993;2:57–71. - PubMed
-
- Pilot T, Miyazaki H. Periodontal conditions in Europe. J Clin Periodontol. 1991;18:353–357. - PubMed
-
- Hall TJ, Chambers TJ. Molecular aspects of osteoclast function. Inflamm Res. 1996;45:1–9. - PubMed
-
- Reddy SV. Regulatory mechanisms operative in osteoclasts. Crit Rev Eukaryot Gene Expr. 2004;14:255–270. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

