Both stereoselective (R)- and (S)-1-Methyl-1,2,3,4-tetrahydroisoquinoline enantiomers protect striatal terminals against rotenone-induced suppression of dopamine release
- PMID: 21069490
- PMCID: PMC3110269
- DOI: 10.1007/s12640-010-9228-5
Both stereoselective (R)- and (S)-1-Methyl-1,2,3,4-tetrahydroisoquinoline enantiomers protect striatal terminals against rotenone-induced suppression of dopamine release
Abstract
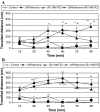
1-Methyl-1,2,3,4-tetrahydroisoquinoline (1MeTIQ) is present in the human and rodent brain as a mixture of stereospecific (R)- and (S)-1MeTIQ enantiomers. The racemate, (R,S)-1MeTIQ, exhibits neuroprotective activity as shown in the earlier study by the authors, and In addition, it was suggested to play a crucial physiological role in the mammalian brain as an endogenous regulator of dopaminergic activity. In this article, we investigated the influence of stereospecific enantiomers of 1MeTIQ, (R)- and (S)-1MeTIQ (50 mg/kg i.p.) on rotenone-induced (3 mg/kg s.c.) behavioral and neurochemical changes in the rat. In behavioral study, in order to record dynamic motor function of rats, we measured locomotor activity using automated locomotor activity boxes. In biochemical studies, we analyzed in rat striatum the concentration of dopamine (DA) and its metabolites: intraneuronal DOPAC, extraneuronal 3-MT, and final HVA using HPLC with electrochemical detection. Otherwise, DA release was estimated by in vivo microdialysis study. The behavioral study has demonstrated that both acute and repeated (3 times) rotenone administration unimportantly depressed a basic locomotor activity in rat. (R)- and (S)-1MeTIQ stereoisomers (50 mg/kg i.p.) produced a modest behavioral activation both in naïve and rotenone-treated rats. The data from ex vivo neurochemical experiments have shown stereospecificity of 1MeTIQ enantiomers in respect of their effects on DA catabolism. (R)-1MeTIQ significantly increased both the level of the final DA metabolite, HVA (by about 70%), and the rate of DA metabolism (by 50%). In contrast to that, (S)-1MeTIQ significantly depressed DOPAC, HVA levels (by 60 and 40%, respectively), and attenuated the rate of DA metabolism (by about 60%). On the other hand, both the enantiomers increased the concentrations of DA and its extraneuronal metabolite, 3-MT in rat striatum. In vivo microdialysis study has shown that repeated but not acute administration of rotenone produced a deep and significant functional impairment of striatal DA release. Both (R)- and (S)- stereospecific enantiomers of 1MeTIQ antagonized rotenone-induced suppression of DA release; however, the effect of (R)-1MeTIQ was more strongly expressed in microdialysis study. In conclusion, we suggest that both chiral isomers of 1MeTIQ offer neuroprotection against rotenone-induced disturbances in the function of dopaminergic neurons and (R,S)-1MeTIQ will be useful as a drug with marked neuroprotective activity in the brain.
Figures






References
-
- Antkiewicz-Michaluk L. Neuroprotective effects of 1-methyl-1,2,3,4-tetrahydroisoquinoline: in vitro and in vivo studies in rats. Pol J Pharmacol. 2004;56(Suppl):179.
-
- Antkiewicz-Michaluk L, Vetulani J. Tetrahydroisoquinolines as endogenous neurotoxins and neuroprotectants. Acta Neurobiol Exp. 2001;61:246.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

