Percutaneous lines for delivering intravenous antibiotics in people with cystic fibrosis
- PMID: 21069703
- PMCID: PMC6481502
- DOI: 10.1002/14651858.CD008243.pub2
Percutaneous lines for delivering intravenous antibiotics in people with cystic fibrosis
Abstract
Background: Percutaneous long lines (long intravenous lines) and short intravenous lines (also termed cannulae) are both used to deliver intravenous antibiotics in cystic fibrosis to treat respiratory exacerbations of the disease. The perceived advantage of a long intravenous line is a greater duration of line function, which has to be balanced against a technically more challenging insertion procedure, and the possibility of more discomfort on insertion.
Objectives: To compare long intravenous lines with short intravenous lines in people with cystic fibrosis receiving intravenous antibiotics, in terms of lifespan of the line, ease of insertion, complication rates of the line and patient satisfaction. This will help patients and clinicians choose between devices.
Search strategy: We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group Trials Register comprising references identified from comprehensive electronic database searches and handsearches of relevant journals and abstract books of conference proceedings.Date of most recent search: 26 August 2010.
Selection criteria: Randomised studies comparing long intravenous lines lines with short intravenous lines or comparing different types of long intravenous lines.
Data collection and analysis: We identified two studies, one comparing long intravenous lines with short intravenous lines, and one comparing two different types of long intravenous lines.
Main results: Two studies (67 participants) were included in the review. Based on the published reports, both studies had potential for bias in several domains. There is some evidence that long intravenous lines are superior to short intravenous lines. One study of 20 participants found that the lifespan of a long intravenous line is longer than that of a short intravenous line, and that participants preferred the long intravenous lines to short intravenous lines. A further study of 47 participants found no difference in lifespan, or participant preference when comparing two different long intravenous lines (the Hydrocath and Vygon EC). Neither study was powered to detect differences in serious complications of the devices.
Authors' conclusions: There is some evidence to support the use of long intravenous lines rather than short intravenous lines, in terms of lifespan of the line and patient satisfaction. There is no evidence to suggest that any one type of long intravenous line is superior, and currently choice of line should be determined by operator and patient preference. There are numerous devices available which are used in cystic fibrosis. Further research is required to identify clinically important differences between these devices.
Conflict of interest statement
None known.
Figures





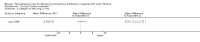
Update of
- doi: 10.1002/14651858.CD008243
Similar articles
-
Antibiotic strategies for eradicating Pseudomonas aeruginosa in people with cystic fibrosis.Cochrane Database Syst Rev. 2017 Apr 25;4(4):CD004197. doi: 10.1002/14651858.CD004197.pub5. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2023 Jun 2;6:CD004197. doi: 10.1002/14651858.CD004197.pub6. PMID: 28440853 Free PMC article. Updated.
-
Insulin and oral agents for managing cystic fibrosis-related diabetes.Cochrane Database Syst Rev. 2016 Apr 18;4:CD004730. doi: 10.1002/14651858.CD004730.pub4. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2020 Oct 19;10:CD004730. doi: 10.1002/14651858.CD004730.pub5. PMID: 27087121 Updated.
-
Oscillating devices for airway clearance in people with cystic fibrosis.Cochrane Database Syst Rev. 2017 May 4;5(5):CD006842. doi: 10.1002/14651858.CD006842.pub4. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Apr 30;4:CD006842. doi: 10.1002/14651858.CD006842.pub5. PMID: 28471492 Free PMC article. Updated.
-
Inhaled mannitol for cystic fibrosis.Cochrane Database Syst Rev. 2018 Feb 9;2(2):CD008649. doi: 10.1002/14651858.CD008649.pub3. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2020 May 1;5:CD008649. doi: 10.1002/14651858.CD008649.pub4. PMID: 29424930 Free PMC article. Updated.
-
Exercise versus airway clearance techniques for people with cystic fibrosis.Cochrane Database Syst Rev. 2022 Jun 22;6(6):CD013285. doi: 10.1002/14651858.CD013285.pub2. Cochrane Database Syst Rev. 2022. PMID: 35731672 Free PMC article.
References
References to studies included in this review
Lacy 1996 {published data only (unpublished sought but not used)}
-
- Lacy DE, Spencer DA, Venkararaman M, Ruiz G, Weller P. Comparison of Two Percutaneous "Midline" Catheters in Cystic Fibrosis. Journal of Intravenous Nursing 1996;16(1):28‐31. - PubMed
References to studies awaiting assessment
Pradeepkumar 1992 {published data only (unpublished sought but not used)}
-
- Pradeepkumar VK, Waseem R, Shortt C, Barry D, Watson JBG. Home intravenous therapy using a silastic long line catheter in cystic fibrosis patients. Irish Medical Journal 1992;85(3):110‐1. - PubMed
Additional references
A‐Rahman 2003
Abedin 2008
-
- Abedin S, Kapoor G. Peripherally inserted central venous catheters are a good option for prolonged venous access in children with cancer. Pediatric Blood & Cancer 2008;5(2):251‐5. - PubMed
Chmiel 2003
Higgins 2003
Higgins 2008a
-
- Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Higgins 2008b
-
- Higgins JPT, Deeks JJ, Altman DG, on behalf of the Cochrane Statistical Methods Group. Chapter 13: Special Topics in Statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Higgins 2008c
-
- Sterne JAC, Egger M, Moher D, on behalf of the Cochrane Bias Methods Group. Chapter 10: Addressing Reporting Biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Jones 2000
-
- Jones SP, Kaslovsky RA. Successful utilization of peripheral inserted central catheters (PICC) for iv antibiotic therapy in pediatric patients with cystic fibrosis (CF) [abstract]. Pediatric Pulmonology 2000;Suppl 20:339.
Lau 2006
Mateu 2002
O'Sullivan 2009
-
- O'Sullivan BP, Freedman SD. Cystic Fibrosis. Lancet 2009;373(9678):1891‐904. - PubMed
Rosenstein 1998
-
- Rosenstein BJ, Cutting GR. The diagnosis of cystic fibrosis: a consensus statement. Cystic Fibrosis Foundation Consensus Panel. Journal of Pediatrics 1998;132(4):589‐95. - PubMed
UK Cystic Fibrosis Trust Antibiotic Working Group
-
- UK Cystic Fibrosis Trust Antibiotic Working Group. Antibiotic Treatment for Cystic Fibrosis. Report of the UK CysticFibrosis Trust Antibiotic Working Group, Third Edition May 2009.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

