PDGF-induced proliferation in human arterial and venous smooth muscle cells: molecular basis for differential effects of PDGF isoforms
- PMID: 21069732
- PMCID: PMC4454503
- DOI: 10.1002/jcb.22924
PDGF-induced proliferation in human arterial and venous smooth muscle cells: molecular basis for differential effects of PDGF isoforms
Abstract
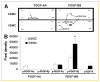
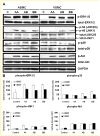
Platelet-derived growth factor (PDGF) has been implicated in the pathogenesis of arterial atherosclerosis and venous neointimal hyperplasia. We examined the effects of PDGF isoforms on smooth muscle cells (SMCs) from arterial and venous origins in order to further understand the differential responsiveness of these vasculatures to proliferative stimuli. Serum-starved human arterial and venous SMCs exhibited very different proliferative responses to PDGF isoforms. Whereas, proliferation of arterial SMCs was strongly stimulated by PDGF-AA, venous SMCs showed no proliferative response to PDGF-AA, but instead demonstrated a significantly greater proliferative response to PDGF-BB than arterial SMCs. Part of this difference could be attributed to differences in PDGF receptors expression. There was a 2.5-fold higher (P < 0.05) density of PDGF receptor-α (PDGF-Rα) and a 6.6-fold lower (P < 0.05) density of PDGF-Rβ expressed on arterial compared to venous SMCs. Concomitant with an increased proliferative response to PDGF-AA in arterial SMCs was a marked PDGF-Rα activation, enhanced phosphorylation of ERK1/2 and Akt, a transient activation of c-Jun NH2-terminal kinase (JNK), and a significant reduction in expression of the cell-cycle inhibitor p27(kip1). This pattern of signaling pathway changes was not observed in venous SMCs. No phosphorylation of PDGF-Rα was detected after venous SMC exposure to PDGF-AA, but there was enhanced phosphorylation of ERK1/2 and Akt in venous SMCs, similar to that seen in the arterial SMCs. PDGF-BB stimulation of venous SMC resulted in PDGF-Rβ activation as well as transactivation of epidermal growth factor receptor (EGF-R); transactivation of EGF-R was not observed in arterial SMCs. These results may provide an explanation for the differential susceptibility to proliferative vascular diseases of arteries and veins.
Figures




Similar articles
-
Differential effects of imatinib on PDGF-induced proliferation and PDGF receptor signaling in human arterial and venous smooth muscle cells.J Cell Biochem. 2006 Dec 15;99(6):1553-63. doi: 10.1002/jcb.20993. J Cell Biochem. 2006. PMID: 16817200
-
Different proliferative properties of smooth muscle cells of human arterial and venous bypass vessels: role of PDGF receptors, mitogen-activated protein kinase, and cyclin-dependent kinase inhibitors.Circulation. 1998 Jan 20;97(2):181-7. doi: 10.1161/01.cir.97.2.181. Circulation. 1998. PMID: 9445171
-
Effects of dominant-negative c-Jun on platelet-derived growth factor-induced vascular smooth muscle cell proliferation.Arterioscler Thromb Vasc Biol. 2002 Jan;22(1):82-8. doi: 10.1161/hq0102.101821. Arterioscler Thromb Vasc Biol. 2002. PMID: 11788465
-
Sodium butyrate inhibits platelet-derived growth factor-induced proliferation of vascular smooth muscle cells.Arterioscler Thromb Vasc Biol. 1995 Dec;15(12):2273-83. doi: 10.1161/01.atv.15.12.2273. Arterioscler Thromb Vasc Biol. 1995. PMID: 7489253
-
Targeting PDGF/PDGFR Signaling Pathway by microRNA, lncRNA, and circRNA for Therapy of Vascular Diseases: A Narrow Review.Biomolecules. 2024 Nov 14;14(11):1446. doi: 10.3390/biom14111446. Biomolecules. 2024. PMID: 39595622 Free PMC article. Review.
Cited by
-
Systematic Analysis of Long Noncoding RNA and mRNA in Granulosa Cells during the Hen Ovulatory Cycle.Animals (Basel). 2021 May 25;11(6):1533. doi: 10.3390/ani11061533. Animals (Basel). 2021. PMID: 34070248 Free PMC article.
-
Platelet secretion: From haemostasis to wound healing and beyond.Blood Rev. 2015 May;29(3):153-62. doi: 10.1016/j.blre.2014.10.003. Epub 2014 Oct 31. Blood Rev. 2015. PMID: 25468720 Free PMC article. Review.
-
Small Molecule Tyrosine Kinase Inhibitor Nintedanib Reduces Development of Cardiac Allograft Vasculopathy in Murine Aortic Allografts.Transplant Direct. 2018 Jun 18;4(7):e367. doi: 10.1097/TXD.0000000000000804. eCollection 2018 Jul. Transplant Direct. 2018. PMID: 30046657 Free PMC article.
-
Potential Molecular Mechanism of Retrograde Aortic Arch Stenosis in the Hybrid Approach to Hypoplastic Left Heart Syndrome.Ann Thorac Surg. 2015 Sep;100(3):1013-9; discussion 1019-20. doi: 10.1016/j.athoracsur.2015.04.125. Epub 2015 Jul 7. Ann Thorac Surg. 2015. PMID: 26163359 Free PMC article.
-
Sequential sequestrations increase the incorporation and retention of multiple growth factors in mineralized collagen scaffolds.RSC Adv. 2020;10(45):26982-26996. doi: 10.1039/d0ra03872e. Epub 2020 Jul 20. RSC Adv. 2020. PMID: 33767853 Free PMC article.
References
-
- Bergsten E, Uutela M, Li X, Pietras K, Ostman A, Heldin CH, Alitalo K, Eriksson U. PDGF-D is a specific, protease-activated ligand for the PDGF beta-receptor. Nat Cell Biol. 2001;3:512–516. - PubMed
-
- Choudhury GG, Grandaliano G, Jin DC, Katz MS, Abboud HE. Activation of PLC and PI 3 kinase by PDGF receptor alpha is not sufficient for mitogenesis and migration in mesangial cells. Kidney Int. 2000;57:908–917. - PubMed
-
- Hachida M, Zhang X, Lu H, Hoshi H, Furutani Y, Matsuoka R, Koyanagi H. Association between the degree of platelet-derived growth factor-A chain mRNA expression and coronary arteriosclerosis in the transplanted heart. Heart Vessels. 1998;13:24–29. - PubMed
-
- Hachida M, Zhang X, Lu H, Hoshi H, Koyanagi H. Effects of immunosuppressants on platelet-derived growth factor-A chain mRNA expression and coronary arteriosclerosis in rat cardiac allografts. Jpn Circ J. 1999;63:303–308. - PubMed
-
- Hackel PO, Zwick E, Prenzel N, Ullrich A. Epidermal growth factor receptors: Critical mediators of multiple receptor pathways. Curr Opin Cell Biol. 1999;11:184–189. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

