A novel non-CB1/TRPV1 endocannabinoid-mediated mechanism depresses excitatory synapses on hippocampal CA1 interneurons
- PMID: 21069781
- PMCID: PMC3117951
- DOI: 10.1002/hipo.20884
A novel non-CB1/TRPV1 endocannabinoid-mediated mechanism depresses excitatory synapses on hippocampal CA1 interneurons
Abstract
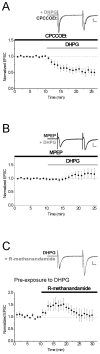
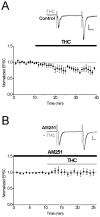
Endocannabinoids (eCBs) mediate various forms of synaptic plasticity at excitatory and inhibitory synapses in the brain. The eCB anandamide binds to several receptors including the transient receptor potential vanilloid 1 (TRPV1) and cannabinoid receptor 1 (CB1). We recently identified that TRPV1 is required for long-term depression at excitatory synapses on CA1 hippocampal stratum radiatum interneurons. Here we performed whole-cell patch clamp recordings from CA1 stratum radiatum interneurons in rat brain slices to investigate the effect of the eCB anandamide on excitatory synapses as well as the involvement of Group I metabotropic glutamate receptors (mGluRs), which have been reported to produce eCBs endogenously. Application of the nonhydrolysable anandamide analog R-methanandamide depressed excitatory transmission to CA1 stratum radiatum interneurons by ∼50%. The Group I mGluR agonist DHPG also depressed excitatory glutamatergic transmission onto interneurons to a similar degree, and this depression was blocked by the mGluR5 antagonist MPEP (10 μM) but not by the mGluR1 antagonist CPCCOEt (50 μM). Interestingly, however, neither DHPG-mediated nor R-methanandamide-mediated depression was blocked by the TRPV1 antagonist capsazepine (10 μM), the CB1 antagonist AM-251 (2 μM) or a combination of both, suggesting the presence of a novel eCB receptor or anandamide target at excitatory hippocampal synapses. DHPG also occluded R-methanandamide depression, suggesting the possibility that the two drugs elicit synaptic depression via a shared signaling mechanism. Collectively, this study illustrates a novel CB1/TRPV1-independent eCB pathway present in the hippocampus that mediates depression at excitatory synapses on CA1 stratum radiatum interneurons.
Copyright © 2010 Wiley Periodicals, Inc.
Figures




Similar articles
-
Endocannabinoids differentially modulate synaptic plasticity in rat hippocampal CA1 pyramidal neurons.PLoS One. 2010 Apr 22;5(4):e10306. doi: 10.1371/journal.pone.0010306. PLoS One. 2010. PMID: 20421986 Free PMC article.
-
NMDA receptors, mGluR5, and endocannabinoids are involved in a cascade leading to hippocampal long-term depression.Neuropsychopharmacology. 2012 Feb;37(3):609-17. doi: 10.1038/npp.2011.243. Epub 2011 Oct 12. Neuropsychopharmacology. 2012. PMID: 21993209 Free PMC article.
-
Endocannabinoid- and mGluR5-dependent short-term synaptic depression in an isolated neuron/bouton preparation from the hippocampal CA1 region.J Neurophysiol. 2008 Aug;100(2):1041-52. doi: 10.1152/jn.90226.2008. Epub 2008 May 21. J Neurophysiol. 2008. PMID: 18497350 Free PMC article.
-
Anandamide-Mediated Modulation of Nociceptive Transmission at the Spinal Cord Level.Physiol Res. 2024 Aug 30;73(S1):S435-S448. doi: 10.33549/physiolres.935371. Epub 2024 Jul 2. Physiol Res. 2024. PMID: 38957948 Free PMC article. Review.
-
Endocannabinoids in striatal plasticity.Parkinsonism Relat Disord. 2012 Jan;18 Suppl 1(Suppl 1):S132-4. doi: 10.1016/S1353-8020(11)70041-4. Parkinsonism Relat Disord. 2012. PMID: 22166411 Free PMC article. Review.
Cited by
-
Inhibitions of anandamide transport and FAAH synthesis decrease apoptosis and oxidative stress through inhibition of TRPV1 channel in an in vitro seizure model.Mol Cell Biochem. 2019 Mar;453(1-2):143-155. doi: 10.1007/s11010-018-3439-0. Epub 2018 Aug 29. Mol Cell Biochem. 2019. PMID: 30159798
-
Sensory TRP channel interactions with endogenous lipids and their biological outcomes.Molecules. 2014 Apr 15;19(4):4708-44. doi: 10.3390/molecules19044708. Molecules. 2014. PMID: 24739932 Free PMC article. Review.
-
Psychiatric Disorders and TRP Channels: Focus on Psychotropic Drugs.Curr Neuropharmacol. 2015;13(2):248-57. doi: 10.2174/1570159x13666150304001606. Curr Neuropharmacol. 2015. PMID: 26411768 Free PMC article. Review.
-
Protease Activated Receptor 2 (PAR2) Induces Long-Term Depression in the Hippocampus through Transient Receptor Potential Vanilloid 4 (TRPV4).Front Mol Neurosci. 2017 Mar 2;10:42. doi: 10.3389/fnmol.2017.00042. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28303089 Free PMC article.
-
Expression of type I mGluRs predicts plasticity in the hippocampal stratum radiatum interneurons.Neurosci Lett. 2019 Nov 1;712:134472. doi: 10.1016/j.neulet.2019.134472. Epub 2019 Sep 6. Neurosci Lett. 2019. PMID: 31499135 Free PMC article.
References
-
- Al-Hayani A, Wease KN, Ross RA, Pertwee RG, Davies SN. The endogenous cannabinoid anandamide activates vanilloid receptors in the rat hippocampal slice. Neuropharmacology. 2001;41(8):1000–1005. - PubMed
-
- Ameri A. The effects of cannabinoids on the brain. Progress in Neurobiology. 1999;58(4):315–348. - PubMed
-
- Auclair N, Otani S, Soubrie P, Crepel F. Cannabinoids Modulate Synaptic Strength and Plasticity at Glutamatergic Synapses of Rat Prefrontal Cortex Pyramidal Neurons. J Neurophysiol. 2000;83(6):3287–3293. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

