Sulfur versus iron oxidation in an iron-thiolate model complex
- PMID: 21070030
- PMCID: PMC3049993
- DOI: 10.1021/ja1045428
Sulfur versus iron oxidation in an iron-thiolate model complex
Abstract
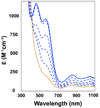
In the absence of base, the reaction of [Fe(II)(TMCS)]PF6 (1, TMCS = 1-(2-mercaptoethyl)-4,8,11-trimethyl-1,4,8,11-tetraazacyclotetradecane) with peracid in methanol at -20 °C did not yield the oxoiron(IV) complex (2, [Fe(IV)(O)(TMCS)]PF6), as previously observed in the presence of strong base (KO(t)Bu). Instead, the addition of 1 equiv of peracid resulted in 50% consumption of 1. The addition of a second equivalent of peracid resulted in the complete consumption of 1 and the formation of a new species 3, as monitored by UV-vis, ESI-MS, and Mössbauer spectroscopies. ESI-MS showed 3 to be formulated as [Fe(II)(TMCS) + 2O](+), while EXAFS analysis suggested that 3 was an O-bound iron(II)-sulfinate complex (Fe-O = 1.95 Å, Fe-S = 3.26 Å). The addition of a third equivalent of peracid resulted in the formation of yet another compound, 4, which showed electronic absorption properties typical of an oxoiron(IV) species. Mössbauer spectroscopy confirmed 4 to be a novel iron(IV) compound, different from 2, and EXAFS (Fe═O = 1.64 Å) and resonance Raman (ν(Fe═O) = 831 cm(-1)) showed that indeed an oxoiron(IV) unit had been generated in 4. Furthermore, both infrared and Raman spectroscopy gave indications that 4 contains a metal-bound sulfinate moiety (ν(s)(SO2) ≈ 1000 cm (-1), ν(as)(SO2) ≈ 1150 cm (-1)). Investigations into the reactivity of 1 and 2 toward H(+) and oxygen atom transfer reagents have led to a mechanism for sulfur oxidation in which 2 could form even in the absence of base but is rapidly protonated to yield an oxoiron(IV) species with an uncoordinated thiol moiety that acts as both oxidant and substrate in the conversion of 2 to 3.
Figures

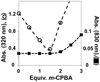



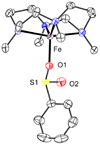
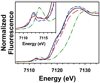
 ), 4 (green,
), 4 (green,  ), and 6 (blue, — — —).
), and 6 (blue, — — —).





Similar articles
-
Activation of Dioxygen by a Mononuclear Nonheme Iron Complex: Sequential Peroxo, Oxo, and Hydroxo Intermediates.J Am Chem Soc. 2019 Nov 6;141(44):17533-17547. doi: 10.1021/jacs.9b05274. Epub 2019 Oct 24. J Am Chem Soc. 2019. PMID: 31647656 Free PMC article.
-
A thiolate-ligated nonheme oxoiron(IV) complex relevant to cytochrome P450.Science. 2005 Nov 11;310(5750):1000-2. doi: 10.1126/science.1119092. Epub 2005 Oct 27. Science. 2005. PMID: 16254150
-
Role of Fe(IV)-oxo intermediates in stoichiometric and catalytic oxidations mediated by iron pyridine-azamacrocycles.Inorg Chem. 2012 May 7;51(9):5006-21. doi: 10.1021/ic202435r. Epub 2012 Apr 25. Inorg Chem. 2012. PMID: 22534174
-
Mössbauer spectroscopy of Fe/S proteins.Biochim Biophys Acta. 2015 Jun;1853(6):1395-405. doi: 10.1016/j.bbamcr.2014.12.005. Epub 2014 Dec 10. Biochim Biophys Acta. 2015. PMID: 25498248 Review.
-
Understanding how the thiolate sulfur contributes to the function of the non-heme iron enzyme superoxide reductase.Acc Chem Res. 2007 Jul;40(7):501-9. doi: 10.1021/ar600059h. Epub 2007 May 31. Acc Chem Res. 2007. PMID: 17536780 Free PMC article. Review.
Cited by
-
Sulfide Oxidation by O2: Synthesis, Structure and Reactivity of Novel Sulfide-Incorporated Fe(II) Bis(imino)pyridine Complexes.Polyhedron. 2013 Jul 13;58:179-189. doi: 10.1016/j.poly.2013.01.043. Polyhedron. 2013. PMID: 23878411 Free PMC article.
-
Di- and Tetrairon(III) μ-Oxido Complexes of an N3S-Donor Ligand: Catalyst Precursors for Alkene Oxidations.Front Chem. 2019 Mar 1;7:97. doi: 10.3389/fchem.2019.00097. eCollection 2019. Front Chem. 2019. PMID: 30881952 Free PMC article.
-
Facile Conversion of syn-[FeIV (O)(TMC)]2+ into the anti Isomer via Meunier's Oxo-Hydroxo Tautomerism Mechanism.Angew Chem Int Ed Engl. 2019 Feb 11;58(7):1995-1999. doi: 10.1002/anie.201811454. Epub 2019 Jan 17. Angew Chem Int Ed Engl. 2019. PMID: 30556289 Free PMC article.
-
A Nonheme Iron(III) Superoxide Complex Leads to Sulfur Oxygenation.J Am Chem Soc. 2024 Mar 27;146(12):7915-7921. doi: 10.1021/jacs.3c12337. Epub 2024 Mar 15. J Am Chem Soc. 2024. PMID: 38488295 Free PMC article.
-
Iron Insertion at the Assembly Site of the ISCU Scaffold Protein Is a Conserved Process Initiating Fe-S Cluster Biosynthesis.J Am Chem Soc. 2022 Sep 28;144(38):17496-17515. doi: 10.1021/jacs.2c06338. Epub 2022 Sep 19. J Am Chem Soc. 2022. PMID: 36121382 Free PMC article.
References
-
- Joseph CA, Moroney MJ. Chem. Commun. 2007:3338–3349. - PubMed
- Aluri S, de Visser SP. J. Am. Chem. Soc. 2007;129:14846–14847. - PubMed
- We S, Wu X, Wei L, Tang D, Sun P, Bartlam M, Rao Z. J. Biol. Chem. 2007;282:3391–3402. - PubMed
- Simmons CR, Krishnamoorthy K, Granett SL, Shuller DJ, Dominy JE, Jr, Begley TP, Stipanuk MH, Karplus PA. Biochemistry. 2008;47:11390–11392. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

