Synthesis and identification of new 4-arylidene curcumin analogues as potential anticancer agents targeting nuclear factor-κB signaling pathway
- PMID: 21070043
- PMCID: PMC3990230
- DOI: 10.1021/jm1004545
Synthesis and identification of new 4-arylidene curcumin analogues as potential anticancer agents targeting nuclear factor-κB signaling pathway
Abstract
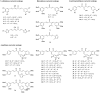
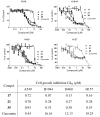
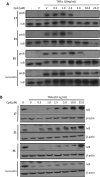
A series of curcumin analogues including new 4-arylidene curcumin analogues (4-arylidene-1,7-bisarylhepta-1,6-diene-3,5-diones) were synthesized. Cell growth inhibition assays revealed that most 4-arylidene curcumin analogues can effectively decrease the growth of a panel of lung cancer cells at submicromolar and low micromolar concentrations. High content analysis technology coupled with biochemical studies showed that this new class of 4-arylidene curcumin analogues exhibits significantly improved NF-κB inhibition activity over the parent compound curcumin, at least in part by inhibiting IκB phosphorylation and degradation via IKK blockage; selected 4-arylidene curcumin analogues also reduced the tumorigenic potential of cancer cells in a clonogenic assay.
Figures


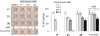


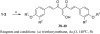
Similar articles
-
Synthesis, cytotoxicity of new 4-arylidene curcumin analogues and their multi-functions in inhibition of both NF-κB and Akt signalling.Eur J Med Chem. 2012 Sep;55:346-57. doi: 10.1016/j.ejmech.2012.07.039. Epub 2012 Jul 31. Eur J Med Chem. 2012. PMID: 22889562
-
Deubiquitinase inhibition of 19S regulatory particles by 4-arylidene curcumin analog AC17 causes NF-κB inhibition and p53 reactivation in human lung cancer cells.Mol Cancer Ther. 2013 Aug;12(8):1381-92. doi: 10.1158/1535-7163.MCT-12-1057. Epub 2013 May 21. Mol Cancer Ther. 2013. PMID: 23696216
-
Inhibition of IkappaB kinase-nuclear factor-kappaB signaling pathway by 3,5-bis(2-flurobenzylidene)piperidin-4-one (EF24), a novel monoketone analog of curcumin.Mol Pharmacol. 2008 Sep;74(3):654-61. doi: 10.1124/mol.108.046201. Epub 2008 Jun 24. Mol Pharmacol. 2008. PMID: 18577686 Free PMC article.
-
Molecular Pathways Modulated by Curcumin Analogue, Diarylpentanoids in Cancer.Biomolecules. 2019 Jul 10;9(7):270. doi: 10.3390/biom9070270. Biomolecules. 2019. PMID: 31295798 Free PMC article. Review.
-
Curcumin and its analogues: potential anticancer agents.Med Res Rev. 2010 Sep;30(5):818-60. doi: 10.1002/med.20188. Med Res Rev. 2010. PMID: 20027668 Review.
Cited by
-
Curcumin analogue T83 exhibits potent antitumor activity and induces radiosensitivity through inactivation of Jab1 in nasopharyngeal carcinoma.BMC Cancer. 2013 Jul 1;13:323. doi: 10.1186/1471-2407-13-323. BMC Cancer. 2013. PMID: 23815987 Free PMC article.
-
Curcumin and its Derivatives Targeting Multiple Signaling Pathways to Elicit Anticancer Activity: A Comprehensive Perspective.Curr Med Chem. 2024;31(24):3668-3714. doi: 10.2174/0929867330666230522144312. Curr Med Chem. 2024. PMID: 37221681 Review.
-
Curcumin: an anti-inflammatory molecule from a curry spice on the path to cancer treatment.Molecules. 2011 Jun 3;16(6):4567-98. doi: 10.3390/molecules16064567. Molecules. 2011. PMID: 21642934 Free PMC article. Review.
-
Preclinical Study of Novel Curcumin Analogue SSC-5 Using Orthotopic Tumor Xenograft Model for Esophageal Squamous Cell Carcinoma.Cancer Res Treat. 2018 Oct;50(4):1362-1377. doi: 10.4143/crt.2017.353. Epub 2018 Jan 24. Cancer Res Treat. 2018. PMID: 29361818 Free PMC article.
-
Antifungal Activity of Curcuminoids and Difluorinated Curcumin Against Clinical Isolates of Candida Species.Adv Exp Med Biol. 2021;1328:123-129. doi: 10.1007/978-3-030-73234-9_8. Adv Exp Med Biol. 2021. PMID: 34981474
References
-
- Ghosh S, May M, Kopp E. NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. - PubMed
-
- Hayden M, Ghosh S. Shared principles in NF-κB signaling. Cell. 2008;132:344–362. - PubMed
-
- Bonizzi G, Karin M. The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. - PubMed
-
- Ni H, Ergin M, Huang Q, Qin J, Amin H, Martinez R, Saeed S, Barton K, Alkan S. Analysis of expression of nuclear factor κB (NF-κB) in multiple myeloma: downregulation of NF-κB induces apoptosis. Br J Haematol. 2001;115:279–286. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

