Efficacy and safety of a new immunoglobulin G product, Gammaplex(®), in primary immunodeficiency diseases
- PMID: 21070209
- PMCID: PMC3026554
- DOI: 10.1111/j.1365-2249.2010.04247.x
Efficacy and safety of a new immunoglobulin G product, Gammaplex(®), in primary immunodeficiency diseases
Abstract
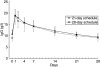
This open-label multi-centre study evaluated a new intravenous immunoglobulin, Gammaplex®, in the treatment of 50 patients with primary immunodeficiency and significant hypogammglobulinaemia. Patients treated previously with other intravenous immunoglobulins received Gammaplex® on their same infusion schedule for 1 year; 22 were on a 21-day and 28 on a 28-day regimen (300-800 mg/kg/infusion). There were no serious, acute bacterial infections, whereas six subjects (12·0%) had at least one such infection in the 6 months before enrollment. Forty subjects (80·0%) had at least one non-serious infection; the median number of infective episodes per subject per year was 3·07. Antibiotics were taken by 38 subjects therapeutically and prophylactically by 16 at some time. Fewer than half (46·0%) missed any time off work or school because of infection or other illness. Trough immunoglobulin (Ig)G levels were above 6·00 g/l in all subjects at all assessments after 15 weeks with two exceptions. Overall, 21·2% of infusions were associated with an adverse event up to 72 h after infusion. The frequency of adverse events increased with infusion rate. Headache was the most common product-related adverse event (7·5% of 703 infusions). In conclusion, Gammaplex® is effective in primary immunodeficiency and is well tolerated.
© 2010 The Authors. Clinical and Experimental Immunology © 2010 British Society for Immunology.
Figures

References
-
- Liese JG, Wintergerst U, Tympner KD, et al. High- vs. low-dose immunoglobulin therapy in the long-term treatment of X-linked agammaglobulinemia. Am J Dis Child. 1992;146:335–9. - PubMed
-
- Roifman CM, Lederman HM, Lavi S, et al. Benefit of intravenous IgG replacement in hypogammaglobulinemic patients with chronic sinopulmonary disease. Am J Med. 1985;79:171–4. - PubMed
-
- Cunningham-Rundles C, Siegal FP, Smithwick EM, et al. Efficacy of intravenous immunoglobulin in primary humoral immunodeficiency disease. Ann Intern Med. 1984;101:435–9. - PubMed
-
- Chapman SA, Gilkerson KL, Davin TD, et al. Acute renal failure and intravenous immune globulin: occurs with sucrose-stabilized, but not with d-sorbitol-stabilzed, formulation. Ann Pharmacother. 2004;38:2059–67. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

