Evolution of CD33-related siglecs: regulating host immune functions and escaping pathogen exploitation?
- PMID: 21070233
- PMCID: PMC3015071
- DOI: 10.1111/j.1365-2567.2010.03368.x
Evolution of CD33-related siglecs: regulating host immune functions and escaping pathogen exploitation?
Abstract
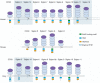
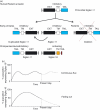
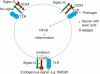
Sialic-acid-binding immunoglobulin-like lectins, siglecs, are important immune receptors expressed widely in mammals. A unique feature of siglecs is their ability to bind sialylated glycans and transmit signals to immune cells. The CD33-related siglecs (CD33rSiglecs) form a major subfamily of the siglecs, containing a large, rapidly evolving group of genes that expanded in mammals through an inverse duplication event involving a primordial cluster of siglec genes over 180 million years ago. Humans express a much larger set of CD33rSiglecs than mice and rats, a feature that can be explained by a dramatic loss of CD33rSiglec genes in rodents. Most CD33rSiglecs have immune receptor tyrosine-based inhibitory motifs and signal negatively. Interestingly, novel DAP-12-coupled 'activating' CD33rSiglecs have been identified, such as siglec-14 and siglec-16, which are paired with the inhibitory receptors, siglec-5 and siglec-11, respectively. The evolution of these activating receptors may have been driven in part by pathogen exploitation of inhibitory siglecs, thereby providing the host with additional pathways by which to combat these pathogens. Inhibitory siglecs seem to play important and varied roles in the regulation of host immune responses. For example, several CD33rSiglecs have been implicated in the negative regulation of Toll-like receptor signalling during innate responses; siglec-G functions as a negative regulator of B1-cell expansion and appears to suppress inflammatory responses to host-derived 'danger-associated molecular patterns'. Recent work has also shown that engagement of neutrophil-expressed siglec-9 by certain strains of sialylated Group B streptococci can suppress killing responses, thereby providing experimental support for pathogen exploitation of host CD33rSiglecs.
Figures
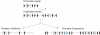
References
-
- Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–66. - PubMed
-
- Varki A, Angata T. Siglecs – the major subfamily of I-type lectins. Glycobiology. 2006;16:1R–27R. - PubMed
-
- Avril T, Attrill H, Zhang J, Raper A, Crocker PR. Negative regulation of leucocyte functions by CD33-related siglecs. Biochem Soc Trans. 2006;34(Pt 6):1024–7. - PubMed
-
- Bashirova AA, Martin MP, McVicar DW, Carrington M. The killer immunoglobulin-like receptor gene cluster: tuning the genome for defense. Annu Rev Genomics Hum Genet. 2006;7:277–300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

