Hippocampal memory consolidation during sleep: a comparison of mammals and birds
- PMID: 21070585
- PMCID: PMC3117012
- DOI: 10.1111/j.1469-185X.2010.00165.x
Hippocampal memory consolidation during sleep: a comparison of mammals and birds
Abstract
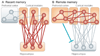
The transition from wakefulness to sleep is marked by pronounced changes in brain activity. The brain rhythms that characterize the two main types of mammalian sleep, slow-wave sleep (SWS) and rapid eye movement (REM) sleep, are thought to be involved in the functions of sleep. In particular, recent theories suggest that the synchronous slow-oscillation of neocortical neuronal membrane potentials, the defining feature of SWS, is involved in processing information acquired during wakefulness. According to the Standard Model of memory consolidation, during wakefulness the hippocampus receives input from neocortical regions involved in the initial encoding of an experience and binds this information into a coherent memory trace that is then transferred to the neocortex during SWS where it is stored and integrated within preexisting memory traces. Evidence suggests that this process selectively involves direct connections from the hippocampus to the prefrontal cortex (PFC), a multimodal, high-order association region implicated in coordinating the storage and recall of remote memories in the neocortex. The slow-oscillation is thought to orchestrate the transfer of information from the hippocampus by temporally coupling hippocampal sharp-wave/ripples (SWRs) and thalamocortical spindles. SWRs are synchronous bursts of hippocampal activity, during which waking neuronal firing patterns are reactivated in the hippocampus and neocortex in a coordinated manner. Thalamocortical spindles are brief 7-14 Hz oscillations that may facilitate the encoding of information reactivated during SWRs. By temporally coupling the readout of information from the hippocampus with conditions conducive to encoding in the neocortex, the slow-oscillation is thought to mediate the transfer of information from the hippocampus to the neocortex. Although several lines of evidence are consistent with this function for mammalian SWS, it is unclear whether SWS serves a similar function in birds, the only taxonomic group other than mammals to exhibit SWS and REM sleep. Based on our review of research on avian sleep, neuroanatomy, and memory, although involved in some forms of memory consolidation, avian sleep does not appear to be involved in transferring hippocampal memories to other brain regions. Despite exhibiting the slow-oscillation, SWRs and spindles have not been found in birds. Moreover, although birds independently evolved a brain region--the caudolateral nidopallium (NCL)--involved in performing high-order cognitive functions similar to those performed by the PFC, direct connections between the NCL and hippocampus have not been found in birds, and evidence for the transfer of information from the hippocampus to the NCL or other extra-hippocampal regions is lacking. Although based on the absence of evidence for various traits, collectively, these findings suggest that unlike mammalian SWS, avian SWS may not be involved in transferring memories from the hippocampus. Furthermore, it suggests that the slow-oscillation, the defining feature of mammalian and avian SWS, may serve a more general function independent of that related to coordinating the transfer of information from the hippocampus to the PFC in mammals. Given that SWS is homeostatically regulated (a process intimately related to the slow-oscillation) in mammals and birds, functional hypotheses linked to this process may apply to both taxonomic groups.
© 2010 The Authors. Biological Reviews © 2010 Cambridge Philosophical Society.
Figures


Similar articles
-
Slow-wave sleep and the consolidation of long-term memory.World J Biol Psychiatry. 2010 Jun;11 Suppl 1:16-21. doi: 10.3109/15622971003637637. World J Biol Psychiatry. 2010. PMID: 20509828 Review.
-
Divergent neuronal activity patterns in the avian hippocampus and nidopallium.Eur J Neurosci. 2020 Aug;52(4):3124-3139. doi: 10.1111/ejn.14675. Epub 2020 Jan 29. Eur J Neurosci. 2020. PMID: 31944434
-
Evolution of slow-wave sleep and palliopallial connectivity in mammals and birds: a hypothesis.Brain Res Bull. 2006 Mar 15;69(1):20-9. doi: 10.1016/j.brainresbull.2005.11.002. Epub 2005 Dec 1. Brain Res Bull. 2006. PMID: 16464681 Review.
-
Intra-"cortical" activity during avian non-REM and REM sleep: variant and invariant traits between birds and mammals.Sleep. 2019 Feb 1;42(2). doi: 10.1093/sleep/zsy230. Sleep. 2019. PMID: 30462347
-
Slow oscillations orchestrating fast oscillations and memory consolidation.Prog Brain Res. 2011;193:93-110. doi: 10.1016/B978-0-444-53839-0.00007-7. Prog Brain Res. 2011. PMID: 21854958 Review.
Cited by
-
Variation in brain regions associated with fear and learning in contrasting climates.Brain Behav Evol. 2012;79(3):181-90. doi: 10.1159/000335421. Epub 2012 Jan 26. Brain Behav Evol. 2012. PMID: 22286546 Free PMC article.
-
About sleep's role in memory.Physiol Rev. 2013 Apr;93(2):681-766. doi: 10.1152/physrev.00032.2012. Physiol Rev. 2013. PMID: 23589831 Free PMC article. Review.
-
Chronic treatment with galantamine rescues reversal learning in an attentional set-shifting test after experimental brain trauma.Exp Neurol. 2019 May;315:32-41. doi: 10.1016/j.expneurol.2019.01.019. Epub 2019 Jan 31. Exp Neurol. 2019. PMID: 30711647 Free PMC article.
-
Neural representations of space in the hippocampus of a food-caching bird.Science. 2021 Jul 16;373(6552):343-348. doi: 10.1126/science.abg2009. Science. 2021. PMID: 34437154 Free PMC article.
-
Light pollution disrupts sleep in free-living animals.Sci Rep. 2015 Sep 4;5:13557. doi: 10.1038/srep13557. Sci Rep. 2015. PMID: 26337732 Free PMC article.
References
-
- Abellán A, Legaz I, Vernier B, Rétaux S, Medina L. Olfactory and amygdalar structures of the chicken ventral pallium based on the combinatorial expression patterns of LIM and other developmental regulatory genes. Journal of Comparative Neurology. 2009;516:166–186. - PubMed
-
- Aboitiz F, Montiel J. Origin and evolution of the vertebrate telencephalon, with special reference to the mammalian neocortex. Advances in Anatomy, Embryology and Cell Biology. 2007;194:1–116. - PubMed
-
- Aboitiz F, Morales D, Montiel J. The evolutionary origin of the mammalian isocortex: Towards an integrated developmental and functional approach. Behavioral and Brain Sciences. 2003;26:535–552. - PubMed
-
- Aguirre GK, Detre JA, Alsop DC, D'Esposito M. The parahippocampus subserves topographical learning in man. Cerebral Cortex. 1996;6:823–829. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

