The G protein regulators EGL-10 and EAT-16, the Giα GOA-1 and the G(q)α EGL-30 modulate the response of the C. elegans ASH polymodal nociceptive sensory neurons to repellents
- PMID: 21070627
- PMCID: PMC2996360
- DOI: 10.1186/1741-7007-8-138
The G protein regulators EGL-10 and EAT-16, the Giα GOA-1 and the G(q)α EGL-30 modulate the response of the C. elegans ASH polymodal nociceptive sensory neurons to repellents
Abstract
Background: Polymodal, nociceptive sensory neurons are key cellular elements of the way animals sense aversive and painful stimuli. In Caenorhabditis elegans, the polymodal nociceptive ASH sensory neurons detect aversive stimuli and release glutamate to generate avoidance responses. They are thus useful models for the nociceptive neurons of mammals. While several molecules affecting signal generation and transduction in ASH have been identified, less is known about transmission of the signal from ASH to downstream neurons and about the molecules involved in its modulation.
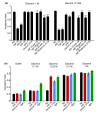
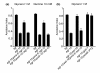
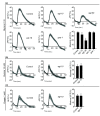
Results: We discovered that the regulator of G protein signalling (RGS) protein, EGL-10, is required for appropriate avoidance responses to noxious stimuli sensed by ASH. As it does for other behaviours in which it is also involved, egl-10 interacts genetically with the G(o)/(i)α protein GOA-1, the G(q)α protein EGL-30 and the RGS EAT-16. Genetic, behavioural and Ca²(+) imaging analyses of ASH neurons in live animals demonstrate that, within ASH, EGL-10 and GOA-1 act downstream of stimulus-evoked signal transduction and of the main transduction channel OSM-9. EGL-30 instead appears to act upstream by regulating Ca²(+) transients in response to aversive stimuli. Analysis of the delay in the avoidance response, of the frequency of spontaneous inversions and of the genetic interaction with the diacylglycerol kinase gene, dgk-1, indicate that EGL-10 and GOA-1 do not affect signal transduction and neuronal depolarization in response to aversive stimuli but act in ASH to modulate downstream transmission of the signal.
Conclusions: The ASH polymodal nociceptive sensory neurons can be modulated not only in their capacity to detect stimuli but also in the efficiency with which they respond to them. The Gα and RGS molecules studied in this work are conserved in evolution and, for each of them, mammalian orthologs can be identified. The discovery of their role in the modulation of signal transduction and signal transmission of nociceptors may help us to understand how pain is generated and how its control can go astray (such as chronic pain) and may suggest new pain control therapies.
Figures
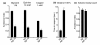


Similar articles
-
Two RGS proteins that inhibit Galpha(o) and Galpha(q) signaling in C. elegans neurons require a Gbeta(5)-like subunit for function.Curr Biol. 2001 Feb 20;11(4):222-31. doi: 10.1016/s0960-9822(01)00071-9. Curr Biol. 2001. PMID: 11250150
-
eat-11 encodes GPB-2, a Gbeta(5) ortholog that interacts with G(o)alpha and G(q)alpha to regulate C. elegans behavior.Curr Biol. 2001 Feb 20;11(4):288-93. doi: 10.1016/s0960-9822(01)00074-4. Curr Biol. 2001. PMID: 11250160 Free PMC article.
-
RSBP-1 is a membrane-targeting subunit required by the Galpha(q)-specific but not the Galpha(o)-specific R7 regulator of G protein signaling in Caenorhabditis elegans.Mol Biol Cell. 2010 Jan 15;21(2):232-43. doi: 10.1091/mbc.e09-07-0642. Epub 2009 Nov 18. Mol Biol Cell. 2010. PMID: 19923320 Free PMC article.
-
Heterotrimeric G proteins in C. elegans.WormBook. 2006 Oct 13:1-25. doi: 10.1895/wormbook.1.75.1. WormBook. 2006. PMID: 18050432 Free PMC article. Review.
-
Genetic analysis of RGS protein function in Caenorhabditis elegans.Methods Enzymol. 2004;389:305-20. doi: 10.1016/S0076-6879(04)89018-9. Methods Enzymol. 2004. PMID: 15313573 Review.
Cited by
-
Dissecting the serotonergic food signal stimulating sensory-mediated aversive behavior in C. elegans.PLoS One. 2011;6(7):e21897. doi: 10.1371/journal.pone.0021897. Epub 2011 Jul 21. PLoS One. 2011. PMID: 21814562 Free PMC article.
-
G protein-coupled receptor-based thermosensation determines temperature acclimatization of Caenorhabditis elegans.Nat Commun. 2024 Feb 23;15(1):1660. doi: 10.1038/s41467-024-46042-z. Nat Commun. 2024. PMID: 38396085 Free PMC article.
-
Parasite neuropeptide biology: Seeding rational drug target selection?Int J Parasitol Drugs Drug Resist. 2011 Nov 15;2:76-91. doi: 10.1016/j.ijpddr.2011.10.004. eCollection 2012 Dec. Int J Parasitol Drugs Drug Resist. 2011. PMID: 24533265 Free PMC article. Review.
-
Structural domains required for Caenorhabditis elegans G protein-coupled receptor kinase 2 (GRK-2) function in vivo.J Biol Chem. 2012 Apr 13;287(16):12634-44. doi: 10.1074/jbc.M111.336818. Epub 2012 Feb 28. J Biol Chem. 2012. PMID: 22375004 Free PMC article.
-
Impacts of chronic low-level nicotine exposure on Caenorhabditis elegans reproduction: identification of novel gene targets.Reprod Toxicol. 2013 Sep;40:69-75. doi: 10.1016/j.reprotox.2013.05.007. Epub 2013 Jun 2. Reprod Toxicol. 2013. PMID: 23735997 Free PMC article.
References
-
- Bargmann CI. In: WormBook. Community eTCeR, editor. 2006. Chemosensation in C. elegans; pp. 1–29.http://www.wormbook.org - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

