Rhizobium leguminosarum bv. trifolii rosR is required for interaction with clover, biofilm formation and adaptation to the environment
- PMID: 21070666
- PMCID: PMC2996380
- DOI: 10.1186/1471-2180-10-284
Rhizobium leguminosarum bv. trifolii rosR is required for interaction with clover, biofilm formation and adaptation to the environment
Abstract
Background: Rhizobium leguminosarum bv. trifolii is a symbiotic nitrogen-fixing bacterium that elicits nodules on roots of host plants Trifolium spp. Bacterial surface polysaccharides are crucial for establishment of a successful symbiosis with legumes that form indeterminate-type nodules, such as Trifolium, Pisum, Vicia, and Medicago spp. and aid the bacterium in withstanding osmotic and other environmental stresses. Recently, the R. leguminosarum bv. trifolii RosR regulatory protein which controls exopolysaccharide production has been identified and characterized.
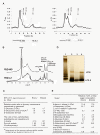
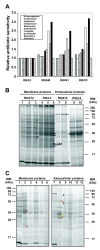
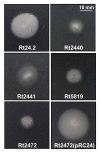
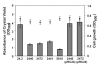
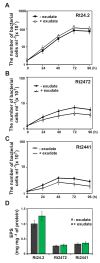
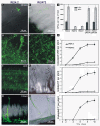
Results: In this work, we extend our earlier studies to the characterization of rosR mutants which exhibit pleiotropic phenotypes. The mutants produce three times less exopolysaccharide than the wild type, and the low-molecular-weight fraction in that polymer is greatly reduced. Mutation in rosR also results in quantitative alterations in the polysaccharide constituent of lipopolysaccharide. The rosR mutants are more sensitive to surface-active detergents, antibiotics of the beta-lactam group and some osmolytes, indicating changes in the bacterial membranes. In addition, the rosR mutants exhibit significant decrease in motility and form a biofilm on plastic surfaces, which differs significantly in depth, architecture, and bacterial viability from that of the wild type. The most striking effect of rosR mutation is the considerably decreased attachment and colonization of root hairs, indicating that the mutation affects the first stage of the invasion process. Infection threads initiate at a drastically reduced rate and frequently abort before they reach the base of root hairs. Although these mutants form nodules on clover, they are unable to fix nitrogen and are outcompeted by the wild type in mixed inoculations, demonstrating that functional rosR is important for competitive nodulation.
Conclusions: This report demonstrates the significant role RosR regulatory protein plays in bacterial stress adaptation and in the symbiotic relationship between clover and R. leguminosarum bv. trifolii 24.2.
Figures



Similar articles
-
Environmental signals and regulatory pathways that influence exopolysaccharide production in rhizobia.Int J Mol Sci. 2011;12(11):7898-933. doi: 10.3390/ijms12117898. Epub 2011 Nov 15. Int J Mol Sci. 2011. PMID: 22174640 Free PMC article. Review.
-
Transcriptome profiling of a Rhizobium leguminosarum bv. trifolii rosR mutant reveals the role of the transcriptional regulator RosR in motility, synthesis of cell-surface components, and other cellular processes.BMC Genomics. 2015 Dec 29;16:1111. doi: 10.1186/s12864-015-2332-4. BMC Genomics. 2015. PMID: 26715155 Free PMC article.
-
Modulation of rosR expression and exopolysaccharide production in Rhizobium leguminosarum bv. trifolii by phosphate and clover root exudates.Int J Mol Sci. 2011;12(6):4132-55. doi: 10.3390/ijms12064132. Epub 2011 Jun 22. Int J Mol Sci. 2011. PMID: 21747729 Free PMC article.
-
Multiple copies of rosR and pssA genes enhance exopolysaccharide production, symbiotic competitiveness and clover nodulation in Rhizobium leguminosarum bv. trifolii.Antonie Van Leeuwenhoek. 2009 Nov;96(4):471-86. doi: 10.1007/s10482-009-9362-3. Epub 2009 Jul 9. Antonie Van Leeuwenhoek. 2009. PMID: 19588265
-
The role of bacterial biofilms and surface components in plant-bacterial associations.Int J Mol Sci. 2013 Jul 30;14(8):15838-59. doi: 10.3390/ijms140815838. Int J Mol Sci. 2013. PMID: 23903045 Free PMC article. Review.
Cited by
-
Environmental signals and regulatory pathways that influence exopolysaccharide production in rhizobia.Int J Mol Sci. 2011;12(11):7898-933. doi: 10.3390/ijms12117898. Epub 2011 Nov 15. Int J Mol Sci. 2011. PMID: 22174640 Free PMC article. Review.
-
Genomic Diversity in the Endosymbiotic Bacterium Rhizobium leguminosarum.Genes (Basel). 2018 Jan 24;9(2):60. doi: 10.3390/genes9020060. Genes (Basel). 2018. PMID: 29364862 Free PMC article.
-
Whole-genome sequencing of Mesorhizobium huakuii 7653R provides molecular insights into host specificity and symbiosis island dynamics.BMC Genomics. 2014 Jun 6;15(1):440. doi: 10.1186/1471-2164-15-440. BMC Genomics. 2014. PMID: 24906389 Free PMC article.
-
The convergent xenogeneic silencer MucR predisposes α-proteobacteria to integrate AT-rich symbiosis genes.Nucleic Acids Res. 2022 Aug 26;50(15):8580-8598. doi: 10.1093/nar/gkac664. Nucleic Acids Res. 2022. PMID: 36007892 Free PMC article.
-
Exopolysaccharide biosynthesis enables mature biofilm formation on abiotic surfaces by Herbaspirillum seropedicae.PLoS One. 2014 Oct 13;9(10):e110392. doi: 10.1371/journal.pone.0110392. eCollection 2014. PLoS One. 2014. PMID: 25310013 Free PMC article.
References
-
- Becker A, Pühler A. In: Rhizobiaceae. Molecular Biology of Plant-Associated Bacteria. Spaink HP, Kondorosi A, Hooykaas PJJ, editor. Kluwer Dordrecht: Academic Press; 1998. Production of exopolysaccharides; pp. 97–118.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

