Synthesis and photochemical transformation of 3β,21-dihydroxypregna-5,7-dien-20-one to novel secosteroids that show anti-melanoma activity
- PMID: 21070794
- PMCID: PMC3005096
- DOI: 10.1016/j.steroids.2010.10.009
Synthesis and photochemical transformation of 3β,21-dihydroxypregna-5,7-dien-20-one to novel secosteroids that show anti-melanoma activity
Abstract
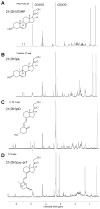
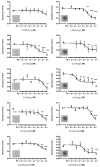
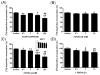
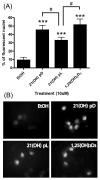
We have synthesized 3β,21-dihydroxypregna-5,7-dien-20-one (21(OH) 7DHP) and used UVB radiation to induce its photoconversion to analogues of vitamin D (pD), lumisterol (pL) and tachysterol (pT). The number and character of the products and the dynamics of the process were dependent on the UVB dose. The main products: pD and pT compounds were characterized by UV absorption, MS and NMR spectroscopy after RP-HPLC chromatography. In addition, formation of multiple oxidized derivatives of the primary products was detected and one of these derivatives was characterized as oxidized 21-hydroxyisotachysterol compound (21(OH)oxy-piT). These newly synthesized compounds inhibited growth of human melanoma cells in a dose dependent manner, with greater or equal potency to calcitriol. 3β,21-Dihydroxy-9β,10α-pregna-5,7-dien-20-one (21(OH)pL) and 21(OH)oxy-piT had higher potency against pigmented melanoma cells, while the EC(50) for compounds 21(OH)7DHP and (5Z,7E)-3β,21-dihydroxy-9,10-secopregna-5,7,10(19)-trien-20-one (21(OH)pD) were similar in both pigmented and non-pigmented cells. Moreover, 21(OH)7DHP and its derivatives inhibited proliferation of human epidermal HaCaT keratinocytes, albeit at a lower activity compared to melanoma cells. Importantly, 21(OH)7DHP derivatives strongly inhibited the colony formation of human melanoma cells with 21(OH)pD being the most potent. The potential mechanism of action of newly synthesized compounds was similar to that mediated by 1,25(OH)(2)D(3) and involved ligand-induced translocation of vitamin D receptor into the nucleus. In summary, we have characterized for the first time products of UVB-induced conversion of 21(OH)7DHP and documented that these compounds have selective, inhibitory effects on melanoma cells.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Photo-conversion of two epimers (20R and 20S) of pregna-5,7-diene-3beta, 17alpha, 20-triol and their bioactivity in melanoma cells.Steroids. 2009 Feb;74(2):218-28. doi: 10.1016/j.steroids.2008.10.017. Epub 2008 Nov 6. Steroids. 2009. PMID: 19028513 Free PMC article.
-
Sequential metabolism of 7-dehydrocholesterol to steroidal 5,7-dienes in adrenal glands and its biological implication in the skin.PLoS One. 2009;4(2):e4309. doi: 10.1371/journal.pone.0004309. Epub 2009 Feb 3. PLoS One. 2009. PMID: 19190754 Free PMC article.
-
Synthesis and photo-conversion of androsta- and pregna-5,7-dienes to vitamin D3-like derivatives.Photochem Photobiol Sci. 2008 Dec;7(12):1570-6. doi: 10.1039/b809005j. Epub 2008 Sep 4. Photochem Photobiol Sci. 2008. PMID: 19037511 Free PMC article.
-
Novel non-calcemic secosteroids that are produced by human epidermal keratinocytes protect against solar radiation.J Steroid Biochem Mol Biol. 2015 Apr;148:52-63. doi: 10.1016/j.jsbmb.2015.01.014. Epub 2015 Jan 21. J Steroid Biochem Mol Biol. 2015. PMID: 25617667 Free PMC article. Review.
-
The role of CYP11A1 in the production of vitamin D metabolites and their role in the regulation of epidermal functions.J Steroid Biochem Mol Biol. 2014 Oct;144 Pt A:28-39. doi: 10.1016/j.jsbmb.2013.10.012. Epub 2013 Oct 28. J Steroid Biochem Mol Biol. 2014. PMID: 24176765 Free PMC article. Review.
Cited by
-
Correlation between secosteroid-induced vitamin D receptor activity in melanoma cells and computer-modeled receptor binding strength.Mol Cell Endocrinol. 2012 Sep 25;361(1-2):143-52. doi: 10.1016/j.mce.2012.04.001. Epub 2012 Apr 21. Mol Cell Endocrinol. 2012. PMID: 22546549 Free PMC article.
-
Lumisterol is metabolized by CYP11A1: discovery of a new pathway.Int J Biochem Cell Biol. 2014 Oct;55:24-34. doi: 10.1016/j.biocel.2014.08.004. Epub 2014 Aug 13. Int J Biochem Cell Biol. 2014. PMID: 25130438 Free PMC article.
-
Key role of CRF in the skin stress response system.Endocr Rev. 2013 Dec;34(6):827-84. doi: 10.1210/er.2012-1092. Epub 2013 Aug 12. Endocr Rev. 2013. PMID: 23939821 Free PMC article. Review.
-
Effects of sidechain length and composition on the kinetic conversion and product distribution of vitamin D analogs determined by real-time NMR.Dermatoendocrinol. 2013 Jan 1;5(1):142-9. doi: 10.4161/derm.24339. Dermatoendocrinol. 2013. PMID: 24494047 Free PMC article.
-
The Role of Classical and Novel Forms of Vitamin D in the Pathogenesis and Progression of Nonmelanoma Skin Cancers.Adv Exp Med Biol. 2020;1268:257-283. doi: 10.1007/978-3-030-46227-7_13. Adv Exp Med Biol. 2020. PMID: 32918223 Free PMC article. Review.
References
-
- Holick MF, Clark MB. The photobiogenesis and metabolism of vitamin D. Fed Proc. 1978;37:2567–74. - PubMed
-
- Holick MF. Vitamin D: A millenium perspective. J Cell Biochem. 2003;88:296–307. - PubMed
-
- Webb AR, DeCosta BR, Holick MF. Sunlight regulates the cutaneous production of vitamin D3 by causing its photodegradation. J Clin Endocrinol Metab. 1989;68:882–7. - PubMed
-
- Tian XQ, Holick MF. A liposomal model that mimics the cutaneous production of vitamin D3. Studies of the mechanism of the membrane-enhanced thermal isomerization of previtamin D3 to vitamin D3. J Biol Chem. 1999;274:4174–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

