Modulation of protein kinase signaling cascades by palytoxin
- PMID: 21070801
- PMCID: PMC3072206
- DOI: 10.1016/j.toxicon.2010.11.003
Modulation of protein kinase signaling cascades by palytoxin
Abstract
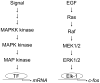
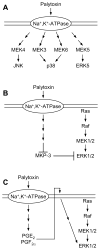
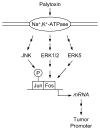
Although known for its acutely toxic action, palytoxin has also been identified as a type of carcinogenic agent called a tumor promoter. In general tumor promoters do not damage DNA, but instead contribute to carcinogenesis by disrupting the regulation of cellular signaling. The identification of palytoxin as a tumor promoter, together with the recognition that the Na(+), K(+)-ATPase is its receptor, led to research on how palytoxin triggers the modulation of signal transduction pathways. This review focuses on mitogen activated protein (MAP) kinases as mediators of palytoxin-stimulated signaling. MAP kinases are a family of serine/threonine kinases that relay a variety of signals to the cellular machinery that regulates cell fate and function. The studies discussed in this review investigated how palytoxin stimulates MAP kinase activity and, in turn, how MAP kinases mediate the response of cells to palytoxin.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Conflict of interest statement
There are no conflicts of interest.
Figures
Similar articles
-
Palytoxin: exploiting a novel skin tumor promoter to explore signal transduction and carcinogenesis.Am J Physiol Cell Physiol. 2007 Jan;292(1):C24-32. doi: 10.1152/ajpcell.00254.2006. Epub 2006 Jul 19. Am J Physiol Cell Physiol. 2007. PMID: 16855216 Free PMC article. Review.
-
Differential activation of mitogen-activated protein kinases by palytoxin and ouabain, two ligands for the Na+,K+-ATPase.Toxicol Appl Pharmacol. 1998 Aug;151(2):377-84. doi: 10.1006/taap.1998.8471. Toxicol Appl Pharmacol. 1998. PMID: 9707514
-
Activation of stress-activator protein kinase/c-Jun N-terminal kinase by the non-TPA-type tumor promoter palytoxin.Cancer Res. 1996 Feb 1;56(3):637-44. Cancer Res. 1996. PMID: 8564984
-
Cell-type-specific activation of p38 protein kinase cascades by the novel tumor promoter palytoxin.Toxicol Appl Pharmacol. 1999 Oct 15;160(2):109-19. doi: 10.1006/taap.1999.8754. Toxicol Appl Pharmacol. 1999. PMID: 10527909
-
Palytoxin acts through Na+,K+-ATPase.Toxicon. 1989;27(11):1171-87. doi: 10.1016/0041-0101(89)90026-3. Toxicon. 1989. PMID: 2575806 Review.
Cited by
-
Ancient Venom Systems: A Review on Cnidaria Toxins.Toxins (Basel). 2015 Jun 18;7(6):2251-71. doi: 10.3390/toxins7062251. Toxins (Basel). 2015. PMID: 26094698 Free PMC article. Review.
-
Pro-inflammatory effects of palytoxin: an in vitro study on human keratinocytes and inflammatory cells.Toxicol Res (Camb). 2016 May 17;5(4):1172-1181. doi: 10.1039/c6tx00084c. eCollection 2016 Jul 1. Toxicol Res (Camb). 2016. PMID: 30090423 Free PMC article.
-
Marine Polyether Phycotoxin Palytoxin Induces Apoptotic Cell Death via Mcl-1 and Bcl-2 Downregulation.Mar Drugs. 2023 Apr 6;21(4):233. doi: 10.3390/md21040233. Mar Drugs. 2023. PMID: 37103372 Free PMC article.
-
Alternative methods for the detection of emerging marine toxins: biosensors, biochemical assays and cell-based assays.Mar Drugs. 2014 Nov 26;12(12):5719-63. doi: 10.3390/md12125719. Mar Drugs. 2014. PMID: 25431968 Free PMC article. Review.
-
Head and neck cancer cells and xenografts are very sensitive to palytoxin: decrease of c-jun n-terminale kinase-3 expression enhances palytoxin toxicity.Mol Cancer. 2013 Feb 14;12:12. doi: 10.1186/1476-4598-12-12. Mol Cancer. 2013. PMID: 23409748 Free PMC article.
References
-
- Abe J, Kusuhara M, Ulevitch RJ, Berk BC, Lee JD. Big mitogen-activated protein kinase 1 (BMK1) is a redox-sensitive kinase. J Biol Chem. 1996;271:16586–16590. - PubMed
-
- Albanell J, Codony-Servat J, Rojo F, Del Campo JM, Sauleda S, Anido J, Raspall G, Giralt J, Rosello J, Nicholson RI, Mendelsohn J, Baselga J. Activated extracellular signal-regulated kinases: association with epidermal growth factor receptor/transforming growth factor alpha expression in head and neck squamous carcinoma and inhibition by anti-epidermal growth factor receptor treatments. Cancer Res. 2001;61:6500–6510. - PubMed
-
- Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991;1072:129–157. - PubMed
-
- Balmain A, Pragnell IB. Mouse skin carcinomas induced in vivo by chemical carcinogens have a transforming Harvey-ras oncogene. Nature. 1983;303:72–74. - PubMed
-
- Bloethner S, Chen B, Hemminki K, Muller-Berghaus J, Ugurel S, Schadendorf D, Kumar R. Effect of common B-RAF and N-RAS mutations on global gene expression in melanoma cell lines. Carcinogenesis. 2005;26:1224–1232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

