The effects of embryonic knockdown of the candidate dyslexia susceptibility gene homologue Dyx1c1 on the distribution of GABAergic neurons in the cerebral cortex
- PMID: 21070838
- PMCID: PMC3010415
- DOI: 10.1016/j.neuroscience.2010.11.002
The effects of embryonic knockdown of the candidate dyslexia susceptibility gene homologue Dyx1c1 on the distribution of GABAergic neurons in the cerebral cortex
Abstract
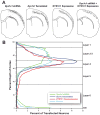
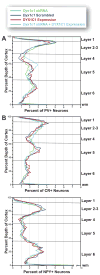
Developmental dyslexia is a language-based learning disability, and a number of candidate dyslexia susceptibility genes have been identified, including DYX1C1, KIAA0319, and DCDC2. Knockdown of function by embryonic transfection of small hairpin RNA (shRNA) of rat homologues of these genes dramatically disrupts neuronal migration to the cerebral cortex by both cell autonomous and non-cell autonomous effects. Here we sought to investigate the extent of non-cell autonomous effects following in utero disruption of the candidate dyslexia susceptibility gene homolog Dyx1c1 by assessing the effects of this disruption on GABAergic neurons. We transfected the ventricular zone of embryonic day (E) 15.5 rat pups with either Dyx1c1 shRNA, DYX1C1 expression construct, both Dyx1c1 shRNA and DYX1C1 expression construct, or a scrambled version of Dyx1c1 shRNA, and sacrificed them at postnatal day 21. The mothers of these rats were injected with BrdU at either E13.5, E15.5, or E17.5. Neurons transfected with Dyx1c1 shRNA were bi-modally distributed in the cerebral cortex with one population in heterotopic locations at the white matter border and another migrating beyond their expected location in the cerebral cortex. In contrast, there was no disruption of migration following transfection with the DYX1C1 expression construct. We found untransfected GABAergic neurons (parvalbumin, calretinin, and neuropeptide Y) in the heterotopic collections of neurons in Dyx1c1 shRNA treated animals, supporting the hypothesis of non-cell autonomous effects. In contrast, we found no evidence that the position of the GABAergic neurons that made it to the cerebral cortex was disrupted by the embryonic transfection with any of the constructs. Taken together, these results support the notion that neurons within heterotopias caused by transfection with Dyx1c1 shRNA result from both cell autonomous and non-cell autonomous effects, but there is no evidence to support non-cell autonomous disruption of neuronal position in the cerebral cortex itself.
Copyright © 2011 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures




Similar articles
-
Position of neocortical neurons transfected at different gestational ages with shRNA targeted against candidate dyslexia susceptibility genes.PLoS One. 2013 May 28;8(5):e65179. doi: 10.1371/journal.pone.0065179. Print 2013. PLoS One. 2013. PMID: 23724130 Free PMC article.
-
Embryonic disruption of the candidate dyslexia susceptibility gene homolog Kiaa0319-like results in neuronal migration disorders.Neuroscience. 2013 Sep 17;248:585-93. doi: 10.1016/j.neuroscience.2013.06.056. Epub 2013 Jul 3. Neuroscience. 2013. PMID: 23831424 Free PMC article.
-
Postnatal analysis of the effect of embryonic knockdown and overexpression of candidate dyslexia susceptibility gene homolog Dcdc2 in the rat.Neuroscience. 2008 Mar 27;152(3):723-33. doi: 10.1016/j.neuroscience.2008.01.020. Epub 2008 Jan 19. Neuroscience. 2008. PMID: 18313856 Free PMC article.
-
Progress towards a cellular neurobiology of reading disability.Neurobiol Dis. 2010 May;38(2):173-80. doi: 10.1016/j.nbd.2009.06.019. Epub 2009 Jul 17. Neurobiol Dis. 2010. PMID: 19616627 Free PMC article. Review.
-
Breakthroughs in the search for dyslexia candidate genes.Trends Mol Med. 2006 Jul;12(7):333-41. doi: 10.1016/j.molmed.2006.05.007. Epub 2006 Jun 16. Trends Mol Med. 2006. PMID: 16781891 Review.
Cited by
-
Dyslexia risk variant rs600753 is linked with dyslexia-specific differential allelic expression of DYX1C1.Genet Mol Biol. 2018 Jan-Mar;41(1):41-49. doi: 10.1590/1678-4685-GMB-2017-0165. Epub 2018 Feb 19. Genet Mol Biol. 2018. PMID: 29473935 Free PMC article.
-
Neural Noise Hypothesis of Developmental Dyslexia.Trends Cogn Sci. 2017 Jun;21(6):434-448. doi: 10.1016/j.tics.2017.03.008. Epub 2017 Apr 8. Trends Cogn Sci. 2017. PMID: 28400089 Free PMC article. Review.
-
Neurobiological bases of reading disorder Part I: Etiological investigations.Lang Linguist Compass. 2017 Apr;11(4):e12239. doi: 10.1111/lnc3.12239. Epub 2017 Apr 23. Lang Linguist Compass. 2017. PMID: 28785303 Free PMC article.
-
Neuroimaging genetics studies of specific reading disability and developmental language disorder: A review.Lang Linguist Compass. 2019 Sep;13(9):e12349. doi: 10.1111/lnc3.12349. Epub 2019 Sep 5. Lang Linguist Compass. 2019. PMID: 31844423 Free PMC article.
-
[Research advances in susceptible genes for developmental dyslexia in children].Zhongguo Dang Dai Er Ke Za Zhi. 2016 Dec;18(12):1308-1312. doi: 10.7499/j.issn.1008-8830.2016.12.022. Zhongguo Dang Dai Er Ke Za Zhi. 2016. PMID: 27974128 Free PMC article. Review. Chinese.
References
-
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. - PubMed
-
- Anthoni H, Zucchelli M, Matsson H, Muller-Myhsok B, Fransson I, Schumacher J, Massinen S, Onkamo P, Warnke A, Griesemann H, Hoffmann P, Nopola-Hemmi J, Lyytinen H, Schulte-Korne G, Kere J, Nothen MM, Peyrard-Janvid M. A locus on 2p12 containing the co-regulated MRPL19 and C2ORF3 genes is associated to dyslexia. Hum Mol Genet. 2007;16:667–677. - PubMed
-
- Bai J, Ramos RL, Ackman JB, Thomas AM, Lee RV, LoTurco JJ. RNAi reveals doublecortin is required for radial migration in rat neocortex. Nat Neurosci. 2003;6:1277–1283. - PubMed
-
- Behar TN, Schaffner AE, Scott CA, Greene CL, Barker JL. GABA receptor antagonists modulate postmitotic cell migration in slice cultures of embryonic rat cortex. Cereb Cortex. 2000;10:899–909. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

