Dietary n-6 PUFA deprivation downregulates arachidonate but upregulates docosahexaenoate metabolizing enzymes in rat brain
- PMID: 21070866
- PMCID: PMC3018563
- DOI: 10.1016/j.bbalip.2010.10.005
Dietary n-6 PUFA deprivation downregulates arachidonate but upregulates docosahexaenoate metabolizing enzymes in rat brain
Abstract
Background: Dietary n-3 polyunsaturated fatty acid (PUFA) deprivation increases expression of arachidonic acid (AA 20:4n-6)-selective cytosolic phospholipase A(2) (cPLA(2)) IVA and cyclooxygenase (COX)-2 in rat brain, while decreasing expression of docosahexaenoic acid (DHA 22:6n-3)-selective calcium-independent iPLA(2) VIA. Assuming that these enzyme changes represent brain homeostatic responses to deprivation, we hypothesized that dietary n-6 PUFA deprivation would produce changes in the opposite directions.
Methods: Brain expression of PUFA-metabolizing enzymes and their transcription factors was quantified in male rats fed an n-6 PUFA adequate or deficient diet for 15weeks post-weaning.
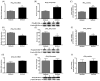
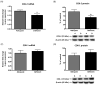
Results: The deficient compared with adequate diet increased brain mRNA, protein and activity of iPLA(2) VIA and 15-lipoxygenase (LOX), but decreased cPLA(2) IVA and COX-2 expression. The brain protein level of the iPLA(2) transcription factor SREBP-1 was elevated, while protein levels were decreased for AP-2α and NF-κB p65, cPLA(2) and COX-2 transcription factors, respectively.
Conclusions: With dietary n-6 PUFA deprivation, rat brain PUFA metabolizing enzymes and some of their transcription factors change in a way that would homeostatically dampen reductions in brain n-6 PUFA concentrations and metabolism, while n-3 PUFA metabolizing enzyme expression is increased. The changes correspond to reported in vitro enzyme selectivities for AA compared with DHA.
Published by Elsevier B.V.
Figures



Similar articles
-
Dietary n-6 polyunsaturated fatty acid deprivation increases docosahexaenoic acid metabolism in rat brain.J Neurochem. 2012 Mar;120(6):985-97. doi: 10.1111/j.1471-4159.2011.07597.x. Epub 2012 Jan 23. J Neurochem. 2012. PMID: 22117540 Free PMC article.
-
Dietary n-3 PUFA deprivation alters expression of enzymes of the arachidonic and docosahexaenoic acid cascades in rat frontal cortex.Mol Psychiatry. 2007 Feb;12(2):151-7. doi: 10.1038/sj.mp.4001887. Epub 2006 Sep 19. Mol Psychiatry. 2007. PMID: 16983392
-
Regulation of rat brain polyunsaturated fatty acid (PUFA) metabolism during graded dietary n-3 PUFA deprivation.Prostaglandins Leukot Essent Fatty Acids. 2011 Dec;85(6):361-8. doi: 10.1016/j.plefa.2011.08.002. Epub 2011 Aug 30. Prostaglandins Leukot Essent Fatty Acids. 2011. PMID: 21880477 Free PMC article.
-
Brain arachidonic and docosahexaenoic acid cascades are selectively altered by drugs, diet and disease.Prostaglandins Leukot Essent Fatty Acids. 2008 Sep-Nov;79(3-5):153-6. doi: 10.1016/j.plefa.2008.09.010. Epub 2008 Oct 29. Prostaglandins Leukot Essent Fatty Acids. 2008. PMID: 18973997 Free PMC article. Review.
-
Imaging brain signal transduction and metabolism via arachidonic and docosahexaenoic acid in animals and humans.Brain Res Bull. 2012 Feb 10;87(2-3):154-71. doi: 10.1016/j.brainresbull.2011.12.001. Epub 2011 Dec 9. Brain Res Bull. 2012. PMID: 22178644 Free PMC article. Review.
Cited by
-
Acyl-CoA synthetase 6 is required for brain docosahexaenoic acid retention and neuroprotection during aging.JCI Insight. 2021 Jun 8;6(11):e144351. doi: 10.1172/jci.insight.144351. JCI Insight. 2021. PMID: 34100386 Free PMC article.
-
Chronic clozapine reduces rat brain arachidonic acid metabolism by reducing plasma arachidonic acid availability.J Neurochem. 2013 Feb;124(3):376-87. doi: 10.1111/jnc.12078. Epub 2012 Dec 6. J Neurochem. 2013. PMID: 23121637 Free PMC article.
-
Lithium and the other mood stabilizers effective in bipolar disorder target the rat brain arachidonic acid cascade.ACS Chem Neurosci. 2014 Jun 18;5(6):459-67. doi: 10.1021/cn500058v. Epub 2014 May 15. ACS Chem Neurosci. 2014. PMID: 24786695 Free PMC article. Review.
-
Disturbed brain phospholipid and docosahexaenoic acid metabolism in calcium-independent phospholipase A(2)-VIA (iPLA(2)β)-knockout mice.Biochim Biophys Acta. 2012 Sep;1821(9):1278-86. doi: 10.1016/j.bbalip.2012.02.003. Epub 2012 Feb 10. Biochim Biophys Acta. 2012. PMID: 22349267 Free PMC article.
-
Fifteen weeks of dietary n-3 polyunsaturated fatty acid deprivation increase turnover of n-6 docosapentaenoic acid in rat-brain phospholipids.Biochim Biophys Acta. 2012 Sep;1821(9):1235-43. doi: 10.1016/j.bbalip.2011.11.002. Epub 2011 Nov 30. Biochim Biophys Acta. 2012. PMID: 22142872 Free PMC article.
References
-
- Farooqui AA, Yi Ong W, Lu XR, Halliwell B, Horrocks LA. Neurochemical consequences of kainate-induced toxicity in brain: involvement of arachidonic acid release and prevention of toxicity by phospholipase A(2) inhibitors. Brain research. 2001;38:61–78. - PubMed
-
- Innis SM. Essential fatty acids in infant nutrition: lessons and limitations from animal studies in relation to studies on infant fatty acid requirements. The American journal of clinical nutrition. 2000;71:238S–244S. - PubMed
-
- Salem N, Jr, Litman B, Kim HY, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 2001;36:945–959. - PubMed
-
- Uauy R, Mena P, Rojas C. Essential fatty acids in early life: structural and functional role. The Proceedings of the Nutrition Society. 2000;59:3–15. - PubMed
-
- Clark JD, Lin LL, Kriz RW, Ramesha CS, Sultzman LA, Lin AY, Milona N, Knopf JL. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca(2+)-dependent translocation domain with homology to PKC and GAP. Cell. 1991;65:1043–1051. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

