Hydrogels with time-dependent material properties enhance cardiomyocyte differentiation in vitro
- PMID: 21071078
- PMCID: PMC3000555
- DOI: 10.1016/j.biomaterials.2010.10.020
Hydrogels with time-dependent material properties enhance cardiomyocyte differentiation in vitro
Abstract
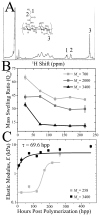
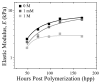
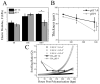
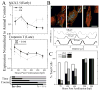
Tissue-specific elastic modulus (E), or 'stiffness,' arises from developmental changes in the extracellular matrix (ECM) and suggests that progenitor cell differentiation may be optimal when physical conditions mimic tissue progression. For cardiomyocytes, maturing from mesoderm to adult myocardium results in a 9-fold stiffening originating in part from a change in collagen expression and localization. To mimic this temporal stiffness change in vitro, thiolated-hyaluronic acid (HA) hydrogels were crosslinked with poly(ethylene glycol) diacrylate, and their dynamics were modulated by changing crosslinker molecular weight. With the hydrogel appropriately tuned to stiffen as heart muscle does during development, pre-cardiac cells grown on collagen-coated HA hydrogels exhibit a 3-fold increase in mature cardiac specific markers and form up to 60% more maturing muscle fibers than they do when grown on compliant but static polyacrylamide hydrogels over 2 weeks. Though ester hydrolysis does not substantially alter hydrogel stiffening over 2 weeks in vitro, model predictions indicate that ester hydrolysis will eventually degrade the material with additional time, implying that this hydrogel may be appropriate for in vivo applications where temporally changing material properties enhance cell maturation prior to its replacement with host tissue.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures



References
-
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. - PubMed
-
- Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23(1):47–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

