Autophosphorylation activates Dictyostelium myosin II heavy chain kinase A by providing a ligand for an allosteric binding site in the alpha-kinase domain
- PMID: 21071445
- PMCID: PMC3024756
- DOI: 10.1074/jbc.M110.177014
Autophosphorylation activates Dictyostelium myosin II heavy chain kinase A by providing a ligand for an allosteric binding site in the alpha-kinase domain
Abstract
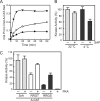
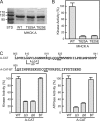
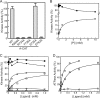
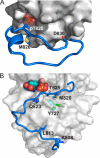
Dictyostelium discoideum myosin II heavy chain kinase A (MHCK A), a member of the atypical α-kinase family, phosphorylates sites in the myosin II tail that block filament assembly. Here we show that the catalytic activity of A-CAT, the α-kinase domain of MHCK A (residues 552-841), is severely inhibited by the removal of a disordered C-terminal tail sequence (C-tail; residues 806-841). The key residue in the C-tail was identified as Thr(825), which was found to be constitutively autophosphorylated. Dephosphorylation of Thr(825) using shrimp alkaline phosphatase decreased A-CAT activity. The activity of a truncated A-CAT lacking Thr(825) could be rescued by P(i), phosphothreonine, and a phosphorylated peptide, but not by threonine, glutamic acid, aspartic acid, or an unphosphorylated peptide. These results focused attention on a P(i)-binding pocket located in the C-terminal lobe of A-CAT. Mutational analysis demonstrated that the P(i)-pocket was essential for A-CAT activity. Based on these results, it is proposed that autophosphorylation of Thr(825) activates ACAT by providing a covalently tethered ligand for the P(i)-pocket. Ab initio modeling studies using the Rosetta FloppyTail and FlexPepDock protocols showed that it is feasible for the phosphorylated Thr(825) to dock intramolecularly into the P(i)-pocket. Allosteric activation is predicted to involve a conformational change in Arg(734), which bridges the bound P(i) to Asp(762) in a key active site loop. Sequence alignments indicate that a comparable regulatory mechanism is likely to be conserved in Dictyostelium MHCK B-D and metazoan eukaryotic elongation factor-2 kinases.
Figures



References
-
- Côte G. P., Bukiejko U. (1987) J. Biol. Chem. 262, 1065–1072 - PubMed
-
- De La Roche M. A., Smith J. L., Betapudi V., Egelhoff T. T., Côté G. P. (2002) J. Muscle Res. Cell Motil. 23, 703–718 - PubMed
-
- Vaillancourt J. P., Lyons C., Côté G. P. (1988) J. Biol. Chem. 263, 10082–10087 - PubMed
-
- Lück-Vielmetter D., Schleicher M., Grabatin B., Wippler J., Gerisch G. (1990) FEBS Lett. 269, 239–243 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

