Continued improvement in myocardial T2* over two years of deferasirox therapy in β-thalassemia major patients with cardiac iron overload
- PMID: 21071497
- PMCID: PMC3012764
- DOI: 10.3324/haematol.2010.031468
Continued improvement in myocardial T2* over two years of deferasirox therapy in β-thalassemia major patients with cardiac iron overload
Abstract
Background: The efficacy of cardiac iron chelation in transfusion-dependent patients has been demonstrated in one-year prospective trials. Since normalization of cardiac T2* takes several years, the efficacy and safety of deferasirox was assessed for two years in patients with β-thalassemia major in the cardiac sub-study of the EPIC trial.
Design and methods: Eligible patients with myocardial T2* greater than 5 to less than 20 ms received deferasirox, with the primary endpoint being the change in T2* from baseline to two years.
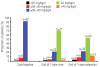
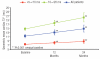
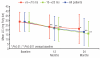
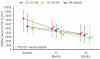
Results: Baseline myocardial T2* was severe (> 5 to < 10 ms) in 39 patients, and moderate-to-mild (10 to < 20 ms) in 62 patients. Mean deferasirox dose was 33.1 ± 3.7 mg/kg/d in the one-year core study increasing to 36.1 ± 7.7 mg/kg/d during the second year of treatment. Geometric mean myocardial T2* increased from a baseline of 11.2 to 14.8 ms at two years (P < 0.001). In patients with moderate-to-mild baseline T2*, an increase was seen from 14.7 to 20.1 ms, with normalization (≥ 20 ms) in 56.7% of patients. In those with severe cardiac iron overload at baseline, 42.9% improved to the moderate-to-mild group. The incidence of drug-related adverse events did not increase during the extension relative to the core study and included (≥ 5%) increased serum creatinine, rash and increased alanine aminotransferase.
Conclusions: Continuous treatment with deferasirox for two years with a target dose of 40 mg/kg/d continued to remove iron from the heart in patients with β-thalassemia major and mild, moderate and severe cardiac siderosis. (Clinicaltrials.gov identifier: NCT 00171821).
Trial registration: ClinicalTrials.gov NCT00171821.
Figures

Comment in
-
In search of the optimal iron chelation therapy for patients with thalassemia major.Haematologica. 2011 Jan;96(1):5-8. doi: 10.3324/haematol.2010.034397. Haematologica. 2011. PMID: 21193427 Free PMC article. No abstract available.
References
-
- Zurlo MG, De Stefano P, Borgna-Pignatti C, Di Palma A, Piga A, Melevendi C, et al. Survival and causes of death in thalassaemia major. Lancet. 1989;2(8653):27–30. - PubMed
-
- Borgna-Pignatti C, Rugolotto S, De Stefano P, Zhao H, Cappellini MD, Del Vecchio GC, et al. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica. 2004;89(10):1187–93. - PubMed
-
- Kolnagou A, Economides C, Eracleous E, Kontoghiorghes GJ. Low serum ferritin levels are misleading for detecting cardiac iron overload and increase the risk of cardiomyopathy in thalassemia patients. The importance of cardiac iron overload monitoring using magnetic resonance imaging T2 and T2*. Hemoglobin. 2006;30(2):219–27. - PubMed
-
- Wood JC, Tyszka JM, Carson S, Nelson MD, Coates TD. Myocardial iron loading in transfusion-dependent thalassemia and sickle cell disease. Blood. 2004;103(5):1934–6. - PubMed

