Claudin-19 and the barrier properties of the human retinal pigment epithelium
- PMID: 21071746
- PMCID: PMC3101667
- DOI: 10.1167/iovs.10-5984
Claudin-19 and the barrier properties of the human retinal pigment epithelium
Abstract
Purpose: The retinal pigment epithelium (RPE) separates photoreceptors from choroidal capillaries, but in age-related macular degeneration (AMD) capillaries breach the RPE barrier. Little is known about human RPE tight junctions or the effects of serum on the retinal side of the RPE.
Methods: Cultured human fetal RPE (hfRPE) was assessed by the transepithelial electrical resistance (TER) and the transepithelial diffusion of methylated polyethylene glycol (mPEG). Claudins and occludin were monitored by quantitative RT-PCR, immunoblotting, and immunofluorescence.
Results: Similar to freshly isolated hfRPE, claudin-19 mRNA was 25 times more abundant than claudin-3. Other detectable claudin mRNAs were found in even lesser amounts, as little as 3000 times less abundant than claudin-19. Claudin-1 and claudin-10b were detected only in subpopulations of cells, whereas others were undetectable. Knockdown of claudin-19 by small interfering RNA (siRNA) eliminated the TER. siRNAs for other claudins had minimal effects. Serum affected tight junctions only when presented to the retinal side of the RPE. The TER increased 2 times, and the conductance of K(+) relative to Na(+) decreased without affecting the permeability of mPEG. These effects correlated with increased steady-state levels of occludin.
Conclusions: Fetal human RPE is a claudin-19-dominant epithelium that has regional variations in claudin-expression. Apical serum decreases RPE permeability, which might be a defense mechanism that would retard the spread of edema due to AMD.
Figures

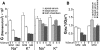


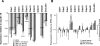




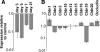


References
-
- Marmor MF, Wolfensberger TJ. The Retinal Pigment Epithelium: Function and Disease. New York: Oxford University Press; 1998
-
- Chang C, Wang X, Caldwell RB. Serum opens tight junctions and reduces ZO-1 protein in retinal epithelial cells. J Neurochem. 1997;69:859–867 - PubMed
-
- Peng S, Rahner C, Rizzolo LJ. Apical and basal regulation of the permeability of the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2003;44:808–817 - PubMed
-
- Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–881 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

