Chemotherapy overcomes TRAIL-R4-mediated TRAIL resistance at the DISC level
- PMID: 21072058
- PMCID: PMC3117243
- DOI: 10.1038/cdd.2010.144
Chemotherapy overcomes TRAIL-R4-mediated TRAIL resistance at the DISC level
Abstract
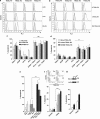
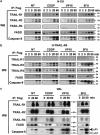
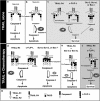
TNF-related apoptosis-inducing ligand or Apo2L (Apo2L/TRAIL) is a promising anti-cancer drug owing to its ability to trigger apoptosis by binding to TRAIL-R1 or TRAIL-R2, two membrane-bound receptors that are often expressed by tumor cells. TRAIL can also bind non-functional receptors such as TRAIL-R4, but controversies still exist regarding their potential to inhibit TRAIL-induced apoptosis. We show here that TRAIL-R4, expressed either endogenously or ectopically, inhibits TRAIL-induced apoptosis. Interestingly, the combination of chemotherapeutic drugs with TRAIL restores tumor cell sensitivity to apoptosis in TRAIL-R4-expressing cells. This sensitization, which mainly occurs at the death-inducing signaling complex (DISC) level, through enhanced caspase-8 recruitment and activation, is compromised by c-FLIP expression and is independent of the mitochondria. Importantly, TRAIL-R4 expression prevents TRAIL-induced tumor regression in nude mice, but tumor regression induced by TRAIL can be restored with chemotherapy. Our results clearly support a negative regulatory function for TRAIL-R4 in controlling TRAIL signaling, and unveil the ability of TRAIL-R4 to cooperate with c-FLIP to inhibit TRAIL-induced cell death.
© 2011 Macmillan Publishers Limited
Figures


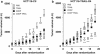
References
-
- Ashkenazi A, Holland P, Eckhardt SG. Ligand-based targeting of apoptosis in cancer: the potential of recombinant human apoptosis ligand 2/Tumor necrosis factor-related apoptosis-inducing ligand (rhApo2L/TRAIL) J Clin Oncol. 2008;26:3621–3630. - PubMed
-
- Berg D, Lehne M, Muller N, Siegmund D, Munkel S, Sebald W, et al. Enforced covalent trimerization increases the activity of the TNF ligand family members TRAIL and CD95L. Cell Death Differ. 2007;14:2021–2034. - PubMed
-
- Ganten TM, Haas TL, Sykora J, Stahl H, Sprick MR, Fas SC, et al. Enhanced caspase-8 recruitment to and activation at the DISC is critical for sensitisation of human hepatocellular carcinoma cells to TRAIL-induced apoptosis by chemotherapeutic drugs. Cell Death Differ. 2004;11 (Suppl 1:S86–S96. - PubMed
-
- Lacour S, Micheau O, Hammann A, Drouineaud V, Tschopp J, Solary E, et al. Chemotherapy enhances TNF-related apoptosis-inducing Ligand DISC assembly in HT29 human colon cancer cells. Oncogene. 2003;22:1807–1816. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

