PPARγ downregulation by TGFß in fibroblast and impaired expression and function in systemic sclerosis: a novel mechanism for progressive fibrogenesis
- PMID: 21072170
- PMCID: PMC2970611
- DOI: 10.1371/journal.pone.0013778
PPARγ downregulation by TGFß in fibroblast and impaired expression and function in systemic sclerosis: a novel mechanism for progressive fibrogenesis
Abstract
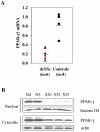
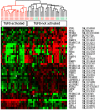
The nuclear orphan receptor peroxisome proliferator-activated receptor-gamma (PPAR-γ) is expressed in multiple cell types in addition to adipocytes. Upon its activation by natural ligands such as fatty acids and eicosanoids, or by synthetic agonists such as rosiglitazone, PPAR-γ regulates adipogenesis, glucose uptake and inflammatory responses. Recent studies establish a novel role for PPAR-γ signaling as an endogenous mechanism for regulating transforming growth factor-ß (TGF-ß)-dependent fibrogenesis. Here, we sought to characterize PPAR-γ function in the prototypic fibrosing disorder systemic sclerosis (SSc), and delineate the factors governing PPAR-γ expression. We report that PPAR-γ levels were markedly diminished in skin and lung biopsies from patients with SSc, and in fibroblasts explanted from the lesional skin. In normal fibroblasts, treatment with TGF-ß resulted in a time- and dose-dependent down-regulation of PPAR-γ expression. Inhibition occurred at the transcriptional level and was mediated via canonical Smad signal transduction. Genome-wide expression profiling of SSc skin biopsies revealed a marked attenuation of PPAR-γ levels and transcriptional activity in a subset of patients with diffuse cutaneous SSc, which was correlated with the presence of a "TGF-ß responsive gene signature" in these biopsies. Together, these results demonstrate that the expression and function of PPAR-γ are impaired in SSc, and reveal the existence of a reciprocal inhibitory cross-talk between TGF-ß activation and PPAR-γ signaling in the context of fibrogenesis. In light of the potent anti-fibrotic effects attributed to PPAR-γ, these observations lead us to propose that excessive TGF-ß activity in SSc accounts for impaired PPAR-γ function, which in turn contributes to unchecked fibroblast activation and progressive fibrosis.
Conflict of interest statement
Figures






Similar articles
-
A synthetic PPAR-γ agonist triterpenoid ameliorates experimental fibrosis: PPAR-γ-independent suppression of fibrotic responses.Ann Rheum Dis. 2014 Feb;73(2):446-54. doi: 10.1136/annrheumdis-2012-202716. Epub 2013 Mar 20. Ann Rheum Dis. 2014. PMID: 23515440 Free PMC article.
-
The adipokine adiponectin has potent anti-fibrotic effects mediated via adenosine monophosphate-activated protein kinase: novel target for fibrosis therapy.Arthritis Res Ther. 2012 Oct 23;14(5):R229. doi: 10.1186/ar4070. Arthritis Res Ther. 2012. PMID: 23092446 Free PMC article.
-
Scleroderma keratinocytes promote fibroblast activation independent of transforming growth factor beta.Rheumatology (Oxford). 2017 Nov 1;56(11):1970-1981. doi: 10.1093/rheumatology/kex280. Rheumatology (Oxford). 2017. PMID: 28968684 Free PMC article.
-
Pathogenesis of scleroderma. Collagen.Rheum Dis Clin North Am. 1996 Nov;22(4):647-74. doi: 10.1016/s0889-857x(05)70294-5. Rheum Dis Clin North Am. 1996. PMID: 8923589 Review.
-
Capturing the heterogeneity in systemic sclerosis with genome-wide expression profiling.Expert Rev Clin Immunol. 2011 Jul;7(4):463-73. doi: 10.1586/eci.11.41. Expert Rev Clin Immunol. 2011. PMID: 21790289 Free PMC article. Review.
Cited by
-
Biological approaches for hypertrophic scars.Int Wound J. 2020 Apr;17(2):405-418. doi: 10.1111/iwj.13286. Epub 2019 Dec 20. Int Wound J. 2020. PMID: 31860941 Free PMC article. Review.
-
Molecular determinants of mesenchymal cell activation in fibroproliferative diseases.Cell Mol Life Sci. 2019 Nov;76(21):4179-4201. doi: 10.1007/s00018-019-03212-3. Epub 2019 Sep 28. Cell Mol Life Sci. 2019. PMID: 31563998 Free PMC article. Review.
-
The PPARα and PPARγ Epigenetic Landscape in Cancer and Immune and Metabolic Disorders.Int J Mol Sci. 2021 Sep 30;22(19):10573. doi: 10.3390/ijms221910573. Int J Mol Sci. 2021. PMID: 34638914 Free PMC article. Review.
-
Interactions between TGF-β1, canonical WNT/β-catenin pathway and PPAR γ in radiation-induced fibrosis.Oncotarget. 2017 Sep 23;8(52):90579-90604. doi: 10.18632/oncotarget.21234. eCollection 2017 Oct 27. Oncotarget. 2017. PMID: 29163854 Free PMC article. Review.
-
Management of Systemic-Sclerosis-Associated Interstitial Lung Disease.Rheum Dis Clin North Am. 2015 Aug;41(3):439-57. doi: 10.1016/j.rdc.2015.04.006. Epub 2015 May 23. Rheum Dis Clin North Am. 2015. PMID: 26210128 Free PMC article. Review.
References
-
- Chen SJ, Yuan W, Mori Y, Levenson A, Trojanowska M, et al. Stimulation of type I collagen transcription in human skin fibroblasts by TGF-beta: involvement of Smad 3. J Invest Dermatol. 1999;112:49–57. - PubMed
-
- Ghosh AK, Yuan W, Mori Y, Varga J. Smad-dependent stimulation of type I collagen gene expression in human skin fibroblasts by TGF-beta involves functional cooperation with p300/CBP transcriptional coactivators. Oncogene. 2000;19:3546–3555. - PubMed
-
- Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

